Potential Protective Effect of Vitamin D on Cardiac Extrinsic pathways of apoptosis in Male Rats Fed with high fat diet
Received: January 14, 2018
Accepted: March 12, 2018
Published: January 16, 2019
Genet.Mol.Res. 18(1):
Keywords
Apoptosis, Heart, Obesity, Vitamin D.
Introduction
Obesity is defined as an excessive amount of body fat in relation to lean mass that compromises healthy individuals (World Health Organization, 2015). It could lead to comorbidities such as type 2 diabetes mellitus, dyslipidaemia’s, hypertension, stroke, and coronary artery disease. (Fitchett, 2014, Chorin et al., 2015). Moreover, obesity has been also well known as an independent risk factor for cardiovascular disease (Hubert et al., 1983). Although it is clear that genetic factors could contribute obesity in an individual, however, the over intake of high calorie food may develop a positive vitality stability and cause the advancement of overweight and obesity condition (Swinburn et al., 2002). There have been conflicting reports related with association of cardiac function and obesity in the scientific literature.
In Saudi Arabia, studies report a constant increase in the prevalence of obesity in the last 3 decades. From late 1980s till mid-1990s, overall prevalence of obesity was estimated at 20% (13.1% in men and 26.6% in women). From 1995 till the last national survey of 2005, in collaboration with world health organization (WHO), all estimates were above 35%. (Kang et al., 2015; Dyson et al., 2014) Since then, national measures have been taken by the Saudi Ministry of Health (SMOH) to control the expansion of the epidemic. However, no further national studies have been conducted to assess the efficacy of these measures, until 2013. (Memish et al., 2014).
In 2013, Memish et al., (2014) conducted a survey with comparable design to the 2005 national survey. They reported significant decrease in the prevalence rates, in comparison with those recorded in the last national survey of 2005 and those estimated for 2010. As per Memish et al., (2014) from 2005 to 2013, men’s and women’s obesity decreased by 4.4% and 10.7%, respectively. Additionally, data from Memish et al., (2014) suggested an increase in the proportion of men and women with normal BMI: + 7.8% and +9.0%, respectively (Memish et al., 2014). However, with regards to age group, data from Memish et al., (2014) demonstrated that the falling trend in prevalence of obesity, from 2005 to 2013, concerned the younger categories of age (<35 years in men and <55 years in women), in comparison with the older categories of ages where the trend was in rise. Regardless this repartition, the significant decrease of obesity in Saudi Arabia is a good reflect of the efficacy of local health measures and policy to control the obesity, and the awareness campaigns undertaken by the SMOH, over the last decade, which focused on dietary and behavioral adjustments (Memish et al., 2014). Human obesity is characterized by low serum 25-hydroxyvitamin D3levels. (Earthman et al., 2012). The correlation between obesity and vitamin D level is unknown. Vitamin D deficiency has been shown to be highly prevalent among general population. and an obviated with various chronic diseases including CVD which might be a leading cause of death. (Assalin et al.,2013).
Even though Saudi Arabia has dry sunny climate all-round the year. The populace barely exposes themselves to direct sunlight. In a study carried out by Ardawi et al, (Ardawi et al., 2012) deficiency of vitamin D was present in 87.8% and vitamin D insufficiency was present in 9.7% in Saudi Arabian men. Also, Aldahr et al, (Aldahar et al.,2011) study was conducted at KAU hospital in jeddah, observed that 80% of children and adolescent’s patients, had vitamin D deficiency while 3.5% of them were vitamin D insufficient. likewise, a study carried out in jeddah by Siddique et al., (2007) that involved 600 adolescent female students revealed low vitamin D levels among 81% and very low levels among 40% of the participants.
Gardner et al., (2011) reported a critical role played by vitamin D in cardiovascular framework. At recent time, heart and vasculature have been recognized as possible focuses of vitamin D activity, a developing assortment of confirmation recommends that the activated VDR take a crucial part in managing cardiovascular activity. (Gardner et al.,2011).Various epidemiological and clinical investigations have revealed a close relationship among hypovitaminosis D and cardiovascular disease (CVD). Scragg et al., (1990) detailed a reverse connection among the levels of serum 25(OH)D and myocardial localized necrosis in a group setting. Martin et al., (2007) revealed a plausible connection amidst hypovitaminosis and main CVD hazard components, such as overweight, hypertension, diabetes, hypertriglyceridemia (however, not hyper-colesterolemia, following alteration for various variables. Moreover, various investigations have demonstrated that hypovitaminosis D is an indicator of cardiovascular and all-cause death, autonomous of the conventional hazard variables. (Wang et al., 2008; Dobnig 2008). Thus, an evaluated reverse connection amid the levels of serum 25(OH)D and CVD is by all accounts most obvious as levels fall beneath 20 to 25 ng/ml. Additionally, Pfeifer et al.,( 2001) observed a decline in blood pressure, heart rate and levels of parathyroid hormone in aged women following interim dispensation of 800 IU of 25(OH)D with 1200 mg calcium against 1200 mg/d calcium singly. This finding indicates that insufficient consumption of 25(OH)D and calcium could assume a conducing part in the pathogenesis and advancement of hypertension and CVD.
Despite several studies showing that a VD-deficient diet induces cardiac changes, the influence of the length of VD restriction on cardiac tissue is unknown. In addition, the mechanisms involved in this process remains to be elucidated.
The present work aimed to investigate the possible protective effect of vitamin D on cardiac Extrinsic pathway of apoptosis in rats fed with HFD for 22 weeks.
Methods
Male wistar rats weighing 150-200 g were housed under standard laboratory conditions at temperature (23 ± 3 0C) and natural 12 hours light/dark cycle with free access of water ad libitum. All animals were kept under conditions that prevent them from experiencing unnecessary pain and discomfort according to guidelines approved by the institutional ethical committee. They were randomly assigned to receive either the standard diet as a normal control rats or high-fat diet rats.
Experimental Protocol
Total 30 rats were included in this study. After one week of acclimatization, the rats were divided into the following groups:
Group I (C ) : The Control rats in this group received the standard diet for six months ( n=5rats).
Group II (C + vit D ): Rats in this group received the standard diet for six months and co-administered with vitamin D by gavaging in a dose of 400 IU/kg/day ( n=5 rats).
Group III (HFD2 ): Rats in this group received a high fat diet (HFD) for six months (n=10 rats)
Group IV (HFD2 + vit D): Rats in this group received a high fat diet (HFD) for six months and co-administered with vitamin D by gavaging in a dose of 400 IU/kg/day ( n=10 rats).
The standard rat chow (D12450 H; research diet center) contains 10 %fat, 70% carbohydrate and 20% protein.
The high Fat diet (D12451; research diet center) contains 45% kcal fat, 35% carbohydrate and 20% protein (Burgmair et al., 2010). Vitamin D were used by gavaging technique in a dose of 400 IU/kg/day (Vieth et al., 2004). The weight and length were measured to asses BMI at the beginning of study and repeated every 45 days. Food intake and body weight were monitored throughout the study period.
At the end of the experimental period, the rats were sacrificed under diethyl ether and the heart were excised and washed with normal saline 0.9% and dried by filter paper. Some heart specimens from different groups were prepared for PCR assessments.
Rna Extraction
Total RNA was isolated from the cells using column purification technology by RNeasy Mini Kit (Qiagen). Separation of RNA was carried out according to the manufacturer ‘s instructions (Qiagen).
Rt-Pcr
Total RNA was reverse transcription of RNA to complementary deoxyribonucleic acid (cDNA) was prepared using cDNA reverse transcription kit (ImProm-II™ Reverse Transcription System cat no. A3800).
Then cDNA amplified by the polymerase chain reaction using the KAPA SYBR® FAST qPCR Kit Master Mix (2X) Universal Cat no. KR0389.
Amplimers were synthesized by MdBio, Inc., based on cDNA sequences from Gen Bank. The rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard. The following rat primers were used: rat FAS forward primer CTGATAGCATCTCTGAGG: rat FAS reverse primer-CTGATAGCATCTCTGAGG; rat FAS-L forward primer-GACAACATAGAGCTGTGG: rat FAS reverse primer- GACAACATAGAGCTGTGG.
Statistical Analysis
Statistical analysis of the data was carried out using SPSS for windows package version 23. (SPSS Inc., Chicago, IL, USA).The data are reported as the mean ± SD except for gene expression results, data are expressed as the mean ± SEM. The results were tested for normality (Shapiro-Wilk test). Data were analyzed by a one way analysis of variance (ANOVA) test to compare between groups. When an interaction was found to be significant, the mean values were compared using multiple comparisons. The Rq values of each gene was compared across all samples of four group using one-tailed student’s t-test with unequal variance to calculate P value for identification of significantly expressed genes. The differences were considered statistically significant if P<0.05.
Results
The results of this study are presented in tables 1 to 3, and illustrated in figures 1 and 2.
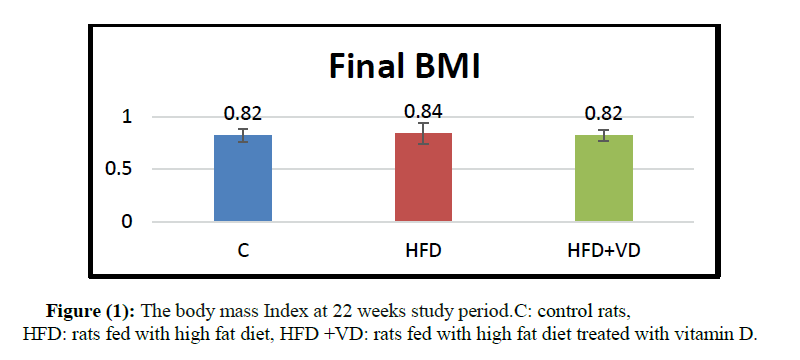
1 - Changes in the body weight, OA length and the BMI:
At the end of the 22 weeks, the body weight, OA length as well as the BMI were not significantly different compared to their controls (table 1and 2) (figure 1).
| Group | Sample No. | Initial Rat weight (gm) | Initial AO length (cm) | BMI (g/cm2) |
Final rat weight (gm) | Final OA length (cm) | BMI (g/cm2) |
|---|---|---|---|---|---|---|---|
| C | 1 | 190 | 21 | 0.4308 | 504 | 25 | 0.8064 |
| 2 | 200 | 21 | 0.4535 | 501 | 25.5 | 0.7705 | |
| 3 | 195 | 19.5 | 0.5128 | 528 | 25.5 | 0.8120 | |
| 4 | 210 | 21.5 | 0.4543 | 605 | 25.5 | 0.9304 | |
| 5 | 212 | 20.52 | 0.5035 | 534 | 26 | 0.7899 | |
| Mean | 201.4 | 20.704 | 0.4710 | 534.4 | 25.5 | 0.8218 | |
| ±SD | 9.4763 | 0.7570 | 0.0350 | 42.0274 | 0.3536 | 0.0628 | |
| C + VD | 6 | 234 | 22.5 | 0.4622 | 533 | 24.5 | 0.8880 |
| 7 | 206 | 20.5 | 0.4902 | 539 | 25 | 0.8624 | |
| 8 | 215 | 21.5 | 0.4651 | 560 | 25.5 | 0.8612 | |
| 9 | 200 | 22 | 0.4132 | 517 | 25 | 0.8272 | |
| 10 | 222 | 21.5 | 0.4803 | 413 | 25.5 | 0.6351 | |
| Mean | 215.4 | 21.6 | 0.4620 | 512.4 | 25.1 | 0.8148 | |
| ±SD | 13.3716 | 0.7416 | 0.0297 | 57.6611 | 0.4183 | 0.1027 | |
| HFD | 11 | 210 | 21.5 | 0.4543 | 521 | 24.5 | 0.8680 |
| 12 | 217 | 21 | 0.4921 | 465 | 24 | 0.8073 | |
| 13 | 220 | 21 | 0.4989 | 586 | 25 | 0.9376 | |
| 14 | 220 | 21.5 | 0.4759 | 481 | 26 | 0.7115 | |
| 15 | 228 | 21.5 | 0.4932 | 509 | 25 | 0.8144 | |
| 16 | 220 | 21.5 | 0.4759 | 455 | 24.5 | 0.7580 | |
| 17 | 230 | 21.5 | 0.4976 | 437 | 25 | 0.6992 | |
| 18 | 216 | 22 | 0.4463 | 564 | 25 | 0.9024 | |
| 19 | 206 | 21 | 0.4671 | 574 | 25.5 | 0.8827 | |
| 20 | 220 | 22 | 0.4545 | 626 | 25 | 1.0016 | |
| Mean | 218.7 | 21.45 | 0.4755 | 521.8 | 24.95 | 0.8383 | |
| ±SD | 7.2119 | 0.3689 | 0.0195 | 63.4154 | 0.5503 | 0.0983 | |
| HFD+VD | 21 | 242 | 22.5 | 0.4780 | 552 | 26 | 0.8166 |
| 22 | 230 | 22.2 | 0.4667 | 487 | 25 | 0.7792 | |
| 23 | 225 | 22.9 | 0.4291 | die | Die | Die | |
| 24 | 205 | 21 | 0.4649 | 568 | 26 | 0.8402 | |
| 25 | 250 | 22.5 | 0.4938 | 560 | 25 | 0.8960 | |
| 26 | 230 | 22.5 | 0.4543 | 518 | 26 | 0.7663 | |
| 27 | 230 | 22.3 | 0.4625 | 550 | 25.5 | 0.8458 | |
| 28 | 240 | 22.7 | 0.4658 | die | Die | Die | |
| 29 | 220 | 21.5 | 0.4759 | 553 | 26 | 0.8180 | |
| 30 | 232 | 22.5 | 0.4583 | 484 | 25 | 0.7744 | |
| Mean | 230.4 | 22.26 | 0.4649 | 534 | 25.563 | 0.8171 | |
| ±SD | 12.4561 | 0.5777 | 0.0169 | 33.2480 | 0.4955 | 0.0438 |
Table 1: Initial and final body weight, OA length and BMI in the different groups of rats treated for 22weeks:
| Groups | Body weight (g) | OA length (cm) | BMI (g/cm2) |
|---|---|---|---|
| C | 534.40± 42.03 | 25.50 ± 0.35 | 0.82 ± 0.06 |
| C +VD | 512 ± 57.66 | 25.10 ± 0.42 | 0.81 ± 0.10 NS |
| HFD | 521.80 ± 63.42 | 24.95 ± 0.55 | 0.84 ± 0.10 NS |
| HFD+VD | 534.00 ± 33.25 | 25.56 ± 0.50 | 0.82 ± 0.04 NS |
C: control rats, C+VD: control rats treated with vitamin D, HFD: rats fed with high fat diet, HFD +VD: rats fed with high fat diet treated with vitamin D. Values are expressed as mean ± SD. Data were analyzed using ANOVA Test. NS: not significant compared to the value of BMI of the control rats C.
Table 2: The body weight, OA length and body mass index at 22 weeks study period.
2- Quantitative Real-time polymerase chain reaction (qRT-PCR):
FAS, FAS-L polypeptides:
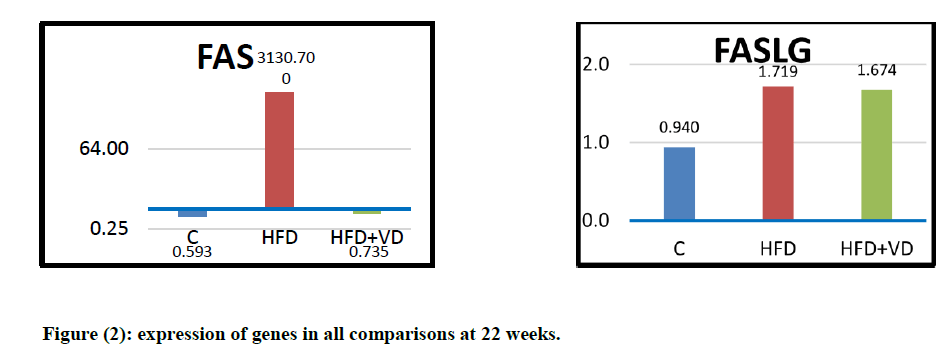
The components of cardiac extrinsic apoptotic signaling pathway were also assessed in the current work. At the end of 22 weeks experimental period, the relative expression of FAS gene was significantly upregulated in high fat fed rats compared with control group and the same applied to FAS-L gene expression. The level of expression of both genes was decreased towards control levels when vitamin D was added to the high fat diet. [Table (3), Figure (2)]
| Cases (Groups) | Genes (Relative Expression) | |
|---|---|---|
| FAS | FASLG | |
| C | 0.593±1.45 | 0.940±1.35 |
| C+VD | 0.742±2.08 | 0.088±1.08 |
| HFD | 3130.7±0.00 | 1.719±1.42 |
| HFD+VD | 0.735±0.73 | 1.674±0.75 |
C=control rats, C+VD= control rats withvitamin D. HFD= rats fed with high fat diet, HFD +VD= rats fed with high fat diet treated with vitamin D. Values are expressed as the mean ± SEM.
Table 3: Relative expression of target genes in each group in
comparison to reference gene,RE = 2 [ΔCT] = 2 [CT reference gene –CT target gene]
Discussion
Over the past decades, there has been a significant increase in the prevalence of nutrition-related diseases, such as obesity, diabetes and cardiovascular diseases. Diet quality may be one of the most important factors associated with hyperglycaemia, hypertriglyceridaemia, hypertension, decreased high-density lipoprotein-cholesterol and increased abdominal circumference (Ramalho et al., 2017). Studies with HFD have been conducted in animals, especially in mice and rats, some of these studies have linked HFD with hyperphagia, weight gain, increased adiposity (Woods et al.,2003), while other studies linked HFD to hyperinsulinemia, insulin resistance without developing obesity (Ramalho et al.,(2017).
The current experiment comprised control group (standard diet 10% fat), HFD group (45% fat), HFD supplemented with vitamin D and a control group that received vitamin D only. At the end of the experiment, no significant difference was observed between groups concerning body weight and BMI. These finding are in consistence with a previous work of Ramalho et al.,(2017), where authors observed that feeding rat HFD (45% fat) for 15 weeks didn’t significantly differ in their body weight from the control group.
In the present study, we observed that HFD feeding increased Fas-L expression in the cardiac tissue that subsequently lead to a significant increase in Fas receptor-dependent apoptotic pathway. The protein levels of cardiac Fas ligand, Fas-associated death domain were significantly increased in the rats fed 45% fat for 12 weeks as stated by Lin et al., (2017). In the current study, vitamin D supplementation with HFD reduced the expression of both Fas and Fas-L towards control levels. The research of Zeng et al., (2017) showed that treatment of rats with induced-diabetic cardiomyopathy with vitamin D down regulated their cardiac Fas and FasL, mRNA and protein levels.
Findings of Zeng et al., together with that of the current study suggest that treatment with vitamin D can potentially reduce the risk of myocardial apoptosis by lowering the Fas and Fas-L expression levels in the heart tissues.
The prominent finding of the present work is that concerning the downregulating effect exerted by vitamin D supplementation on cardiac expression of FAS and FAS-L in chronic high fat feeding model.
Conclusion and Recommendation
The present findings confirm the negative impact of high fat diet ingestion on cardiomyocytes; it enhances myocardial independent pathways of apoptosis.
The current observations expanded our understanding of the cardiac beneficial effects of vitamin D supplementation against chronic consumption of HFD and highlighted an emerging possible role of vitamin D.
Future studies are required to further investigate the possible cardiac protective mechanisms of vitamin D against long term high fat diet consumption.
Conflicts of interest
The authors declare no conflict of interest.
About the Authors
Corresponding Author
Khadeejah Mesaid Al-Solami
Department of Physiology, Faculty of medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- Email:
- khadeejahalsolami@gmail.com
References
- Aldahar S.,Mohammad H.,Abdul-moein E.,Ahmad A.,Abdul-mohsin M.S., Ahmadjee and sendi H.� (2011). Prevelence of vitamin D deficiency and insufficiency in children and adolescents:four year experience. Australian Journal of Basic and Applied Sciences, 5(11): 1616-1620.https://www.researchgate.net/publication/288375386
- Ardawi M, Sibiani A, Bakhsh T, Qari M, et al. (2012). High prevalence of vitamin Deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporosis Int 23:675-686. https://doi.org/10.1007/s00198-011-1606-1
- Assalin HA, Rafacho B, Santos P, Ardisson L (2013). Impact of the length of vitamin D Deficiency on cardiac Remodeling. Circ Heart Fail. 6:809-816. https://doi.org/10.1161/circheartfailure.112.000298
- Burgmaier M, Sen S, Philip F (2010). Metabolic adaptation follows contractile dysfunction in the heart of obese Zucker rats fed a high-fat "Western" diet. Obesity (Silver Spring, Md.). 18(10):1895-901. https://doi.org/10.1038/oby.2009.500
- Chorin E, Hassidim A, Hartal M, Havakuk O, Flint N, Ziv-Baran T, Arbel Y.( 2015 ). Trends in Adolescents Obesity and the Association between BMI and Blood Pressure: A Cross-Sectional Study in 714,922 Healthy Teenagers. Am J Hypertens. 28(9):1157-1163. https://doi.org/10.1093/ajh/hpv007.
- Dyson J, Jaques B, Chattopadyhay D, Lochan R, et al. (2014). Hepatocellular cancer: the impact of obesity, type 2 diabetes, and a multidisciplinary team. J Hepatol. 60(1):110-117. https://doi.org/10.1016/j.jhep.2013.08.011
- Earthman,C.P., Beekman L.M., Kmasodkarand, Dsiblay S., (2012). The link between obesity and low circulating 25-(OH)D concentration: consideration and implications. International journal of obesity 36,387-396.https://doi.org/10.1038/ijo.2011.119
- Fitchett D.( 2014 ). The Metabolic Syndrome Is an Important Concept in Therapeutic Decision-Making. Can J Cardiol. Nov 6. pii: S0828-282X(14)01563-3. https://doi.org/10.1016/j.cjca.2014.10.037
- Garcia-Labb�© D, Ruka E, Bertrand OF, Voisine P, Costerousse O,( 2015 ). Poirier P. Obesity and Coronary Artery Disease: Evaluation and Treatment. Can J Cardiol. Feb;31(2):184-194. https://doi.org/10.1016/j.cjca.2014.12.008
- Gardner DG, Chen S, Glenn DJ, Ni W (2011). Vitamin D and the Cardiovascular System. In: Vitamin D, 3rd Edition. D.J.W. Feldman, Pike and J.S. Adams, eds: 541-563. Elsevier: New York. https://doi.org/10.1016/B978-0-12-381978-9.10031-9
- Kang JH, Kang JY (2015). Lifestyle measures in the management of gastro-esophageal reflux disease: clinical and pathophysiological considerations. Ther Adv Chronic Dis. 6(2):51-64. https://doi.org/10.1177/2040622315569501
- Lin YY, Hsieh PS,Cheng YJ,Cheng SM,Chen CJ,>Huang CY ,Kuo CH,Kao CL,Shyu WC,Lee SD (2017). Anti-apoptotic and Pro-survival Effects of Food Restriction on High-Fat Diet-Induced Obese Hearts.Cardiovasc Toxicol.17(2):163-174.https://doi.org/10.1007/s12012-016-9370-2
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R,( 2007). Norris K: Preva- lence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data From the Third National Health and Nutrition Examination Survey.Arch Intern Med. 167(11):1159-1165.https://doi.org/10.1001/archinte.167.11.1159
- Memish ZA, Bcheraoui C, Tuffaha M, Robinson M, et al. (2014). Obesity and associated factors--Kingdom of Saudi Arabia. Prev Chronic Dis. 11:E174.Nutrition Examination Survey.Arch Intern Med 167:1159-1165. https://doi.org/10.5888/pcd11.140236
- Pfeifer M, Begerow B, Minne HW, Nachtigall D, et al. (2001).� Effects of a short-term vitamin D (3) and calcium supple- mentation on blood pressure and parathyroid hormone. levels in elderly women. J Clin Endocrinol Metab. 86:1633-1637. https://doi.org/10.1210/jc.86.4.1633
- Ramalho L, Jornada M N da, Antunes L C, Hidalgo M P.(2017). Metabolic disturbances due to a high-fat diet in a non-insulin-resistant animal model. Nutrition and Diabetes. 7(3):e245.https://doi.org/10.1038/nutd.2016.47
- Scragg R, Jackson R, Holdaway IM, Lim T, et al. (1990). Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: A community-based study. Int J Epidemiol 19: 559-563. https://doi.org/10.1093/ije/19.3.559
- Siddiqui A, Kamfar H (2007). Prevalence of vitamin D deficiency rickets in adolescent school girls in western region, Saudi Arabia.Saudi Med J. Vol. 28 (3): 441-444. https://doi.org/10.4103/0256-4947.55308
- Swinburn B, Egger G (2002). Preventive strategies against weight gain and obesity. Obes Rev. 3:289-301. https://doi.org/10.1046/j.1467-789x.2002.00082.x
- Vieth R (2004). Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. The Journal of Steroid Biochemistry and Molecular Biology. 89-90, 575-579.https://doi.org/10.1016/j.jsbmb.2004.03.038
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, et al. (2008). Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503-511.https://doi.org/ 10.1161/CIRCULATIONAHA.107.706127
- Woods SC,Seeley RJ,Rushing PA, D'Alessio D, Tso P.(2003). A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 133(4):1081-7. https://doi.org/10.1093/jn/133.4.1081
- World Health Organization (2015). Obesity. Available at:http://www.who.int/topics/obesity/en/ . Last Accessed 11 Mar 2015
- Zeng X, Yu X, Xia S, Yao H (2017). Effect of 1,25-dihydroxyvitamin D3on pathological changes in rats with diabetic cardiomyopathy. Lipid in health and disease.16:109.https://doi.org/10.1186/s12944-017-0498-2
Keywords:
Download:
Full PDF- Share This