The effect of EPS to LPS injure RAW264.7 cell NF-κB and MAPK signaling pathway
Received: October 14, 2017
Accepted: November 08, 2017
Published: December 15, 2017
Genet.Mol.Res. 16(4): gmr16039846
DOI: 10.4238/gmr16039846
Abstract
This study effects of Echinacea polysaccharide(EPS) to Lipopolysaccharides(LPS) injure RAW264.7 cell NF-κB and MAPK signaling pathway. The test was divided into blank groupã€ÂLPS group and 100 mu g/mL EPS + LPS group, The respectively extract Cytoplasm protein and nucleoprotein. After Gel electrophoresis, the Western Blot detected NF-κB, p-p65, IκB-α, p-IκB-α, JNK/p-JNK, ERK/p-ERK, p38/p-p38 several protein expressions. The Results showed that when LPS stimulate RAW264.7, the NF-κB, p-p65, p-IκB-α protein expression increased within the nucleus. Compared with the LPS group, and at 3h, 6h EPS group can effectively restrain increase of NF-κBp65, at 1h, 3h, 6h to restrain expression increases of NF-κB, p-p65 protein expression. At 1h, 3h, EPS group can effectively restrain decrease of IκB-α protein expression (p﹤0.05) and p-IκB-α protein expression increase (p﹤0.05) at 6h. It showed that EPS inhibiting inflammatory reaction and inhibit nuclear factor protein expression. When LPS stimulate RAW264.7, the JNK, ERK, p38 protein expression quantity obviously decreased with longer duration of action at 1h, 3h, 6h. and p-JNK, p-ERK, p-p38 protein expression quantity increased significantly. EPS group JNK, ERK, p38 protein expression were higher than in LPS group. But the p-JNK, p-ERK, p-p38 express quantity significantly reduced. It indicated that EPS inhibition of inflammatory response was associated with activation MAPK signaling pathway
Introduction
Inflammation is a normal physiologic protective reaction of the body through activates the immune system to against pathogen-induced infection and harmful stimuli induced tissue injury (Hagemann et al., 2007). However, inflammation also is a “double-edged sword”. Chronic inflammation was associated with pathological process of several diseased conditions or disorders such as asthma, rheumatoid, arthritis, atherosclerosis, Alzheimer’s disease and cancer (Krishnamoorthy and Honn, 2006). As an important component of the Gram-negative bacterial cell wall, lipopolysaccharide (LPS) can induce inflammatory responses and lead to the disturbance in immune system function (Lou, T. et al. 2015). Intratracheal administration of LPS has gained wide acceptance as a clinically relevant model of severe lung injury (Tao, W. et al. 2015). Nuclear factor-kappaB (NF-kB), a nuclear transcription factor, is a regulator of inflammatory processes. Former studies reported that NF-kB played an important role in the pathogenesis of lung diseases (Bao S, et al. 2015). NF-kB governs maximal transcription of numerous cytokines including tumor necrosis factor- (TNF-a), interleukin (IL)-1, and IL-6 (Li Y. et al. 2015). The inhibition of NF-kB might be a useful strategy for the intervention of inflammatory disease (Wei D, et al. 2014). EPS has anti-inflammatory and immune regulating effect to macrophages. At the same time, A lot of cytokines Participate in the immune regulation and inflammatory response of the body. Our study on the adjustment of EPS to related cytokines and the signaling pathways control. of NF-κB and MAPK in RAW264.7 cell.
Materials and Methods
Materials
LPS (1 mg/ml, ITS-X, Gibco), E. coli O57:B8 (Sigma-Aldrich, St. Louis, MO, USA),RAW 264.7 (Gibco, Grand Island, NY, USA), PMSF (0.1 mg/ml, ITS-X, Gibco), Coomassie brilliant blueR-250 (Peprotech, Rocky Hill, NJ, USA), DMEM (Sigma-Aldrich, St. Louis, MO, USA), TEMED (Nacalai Tesque Inc., Kyoto, Japan), SDS-PAGE (Peprotech, Rocky Hill, NJ, USA), Marker (Gibco, Grand Island, NY, USA), JNK/p-JNK (Gibco, Grand Island, NY, USA),ERK/p-ERK (R&D Systems Inc., Minneapolis, MN, USA), p38/p-p38 (R&D Systems Inc., Minneapolis, MN, USA), NF-κBp65 (R&D Systems Inc., Minneapolis, MN, USA), NF-κBp-p65 (R&D Systems Inc., Minneapolis, MN,USA), IκB-α (Gibco, Grand Island, NY,USA), p-IκB-α (Gibco, Grand Island, NY, USA), β-actin (ITS-X, Gibco).
Electronic balance FA2004B (Bio-Rad, USA), Isotacho-electrophoresis apparatus DYY-6C (Sigma-Aldrich, St. Louis, MO, USA), Electrophoresis chamber (Nacalai Tesque Inc., Kyoto, Japan). HH-601 Super thermostatic water bath (Bio-Rad, USA), High speed refrigerated centrifuge (Nacalai Tesque Inc., Kyoto, Japan), V3 immune imprinting platform thermostatic metal bath (Sigma-Aldrich, St. Louis, MO, USA).
Methods
RAW264.7 cells culture
RAW 264.7 murine macrophage cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were maintained in RPMI 1640 medium supplemented 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in a humidified incubator (model 3110; Forma Scientific, Inc., Marietta, OH, USA) with 5% CO2 at 37°C.
Cells were plated onto 6-cm-diameter type collagen 1-coated dishes (1 × 106 cells per dish). A total of 6 groups of RAW264.7 cells were selected for this study, and each group included six 6 cm-diameter type collagen-coated dishes. The Blank control groups received the following volumes of pbs buffer solution 1 ml, EPS groups received the following volumes of EPS (100 μg/ml) 1 ml, and EPS+LPS groups received the following volumes of EPS (100 μg/ml) + LPS (1 ng/ml) 1 ml. The Cells were cultured for 1 h, 3 h, 6 h and harvested to extract total protein.
Cytoplasmic protein and cytokaryosin extraction
Refer to the cell nucleoprotein extraction kit, the procedure is as follows:Collect cells, count, and washed three times by PBS, each time 14000 rpm centrifuge 3 min. Added 1 ml of reagent A to the1 × 106 cells (added 10 μL PMSF to 1 ml of reagent A). After added reagent A, fully mixed, and placed on ice for 20 min. Added 55 μL reagent B, fully mixed and placed on ice for 1min. 14000rpm, 4°C centrifugal 15 min, removed the supernatant. In precipitation, added 500 μL reagents C (added 5 μL PMSF to 500 μL of reagent C), fully mixed and placed on ice for 40 min, the 10 min vibration time, 14000 rpm, 4°C for 15 min, and Collected supernatant, at -80°C stored.
Western blotting
Protein sample preparation and protein concentration measurement
To 1 × 106 RAW264.7 cells, 500 μL 4°C lysate was added and incubated at 0°C for 30 min. The solution was centrifuged at 12,000 rpm at 4°C for 5 min to obtain the total protein solute. Next, a 20-μL protein sample was placed in a cellulose acetate membrane, dried at 37°C, and dyed with amido black for 5 min. Bleaching occurred by liquid decolorization oscillation three times, for 5 min each time. The membrane was completely dissolved in DMSO, and sample absorbance was read at a 630-nm wavelength. Each sample was replicated three times.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
The 10% separation gel was slowly poured into the glue groove. Water and saturated n-butyl alcohol was then added, and the gel was incubated at room temperature for 2 h. The upper aqueous phase was discarded, and the gel was rinsed three times in double-distilled water. The comb was inserted, 6% concentrated gel was added, and incubated at room temperature for 20 min. The comb was removed, samples were rinsed three times in double-distilled water, and 20 μg electrophoresis samples were loaded in the sample groove. The initial electrophoresis voltage was 80 V, when indicators moved into the separation gel, the voltage was increased to 120 V, and electrophoresis continued until the indicator reached the end of the gel.
Transfer film
The gel plate was removed, transferred to a Petri dish, transfer buffer was added, and incubated for 30 min. At the same time, the nitrocellulose membrane was immersed in transfer buffer and incubated for 20 min. The plate and nitrocellulose membrane were assembled into a “sandwich” structure and placed in the electrophoresis groove. Electrophoresis was run at 4°C for 12 h under an electric current of 150 mA.
Immunization coloration
After electrophoretic transfer, the nitrocellulose membrane was rinsed with PBS. Tween 20 three times for 5 min each time, immersed in 2% BSA, incubated for 30 min, and rinsed another three times in PBS/Tween20 for 5 min each time. Finally, the membranes were blocked with 5% (w/v) non-fat milk in TBST buffer for 1 h at room temperature and incubated with the corresponding primary antibodies overnight at 4°C. After washing, the blots were incubated with horseradish peroxidase conjugated secondary antibodies.
Positive band analysis
Positive band analysis was accomplished using BenQ scanner scan coloration bands, with the Band Scan5.0 software, to analyze protein concentration.
Statistical analysis
Data were collected using a minimum of three experiments and used to calculate the mean ± SD. Statistical differences were analyzed by one-way analysis of variance (ANOVA) followed by multiple comparisons performed with post hoc Bonferroni test (SPSS version 18, John Wiley & Sons, Inc., New York, NY, USA). Values of p<0.05 were considered statistically significant. The significance of any differences between two groups was tested using paired-samples t-test.
Results
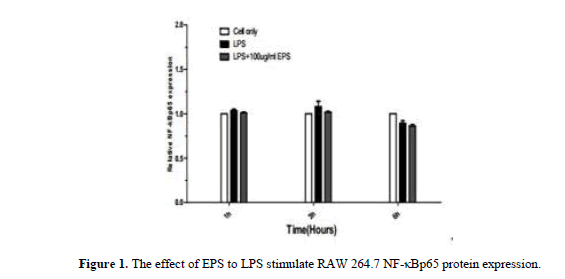
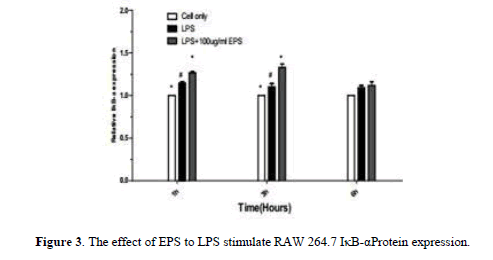
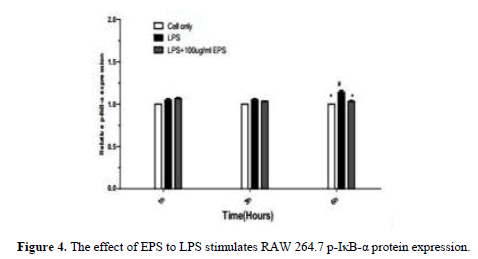
When the LPS stimulated RAW264.7 that NF-κBp65, NF-κBp-p65, p-IκB-α protein expression increases in the nucleus. Compared with LPS group, EPS group can effectively inhibit the expression of NF-κB p65 protein at 3 h and 6 h. The decrease of IκB-α protein expression was effectively inhibited by EPS group at 1 h and 3 h (P<0.05). At 6 h, the expression of p-IκB-α protein was inhibited (P<0.05).The expression of EPS inhibition of inflammation was related to the expression of NF-κB protein (Figures 1-4).
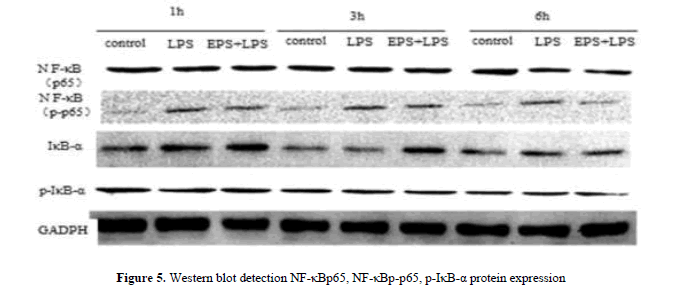
When the LPS stimulated RAW264.7 that NF-κBp65, NF-κBp-p65, IκB-α, p-IκB-α protein expression increases in the nucleus. Compared with LPS group, the expression of NF-κBp65 protein was inhibited by EPS group at 3 h and 6 h, At 1 h, 3 h and 6 h, the expression of NF-κBp-p65 protein was inhibited; The effective inhibited IκB-α protein expression at 1 h and 3 h, and inhibited effectively p-IκB-α protein expression at 6 h. The expression of EPS inhibition of inflammation was related to the expression of NF-κB protein (Figure 5).
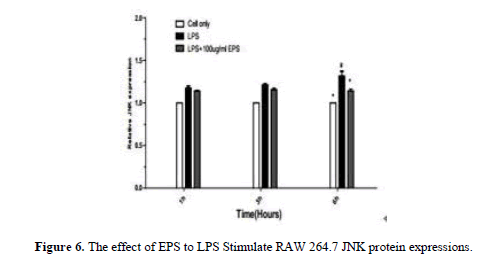
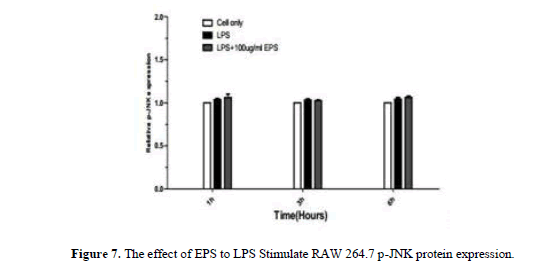
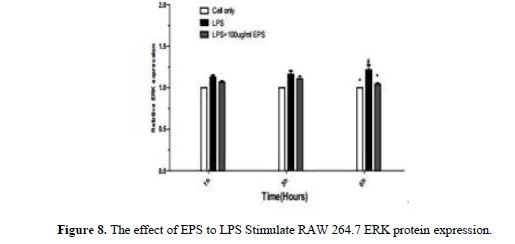
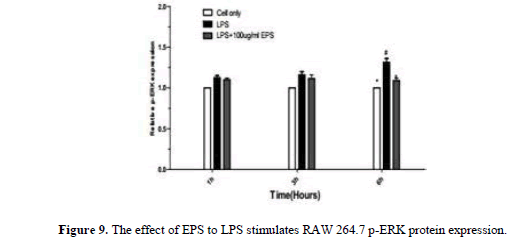
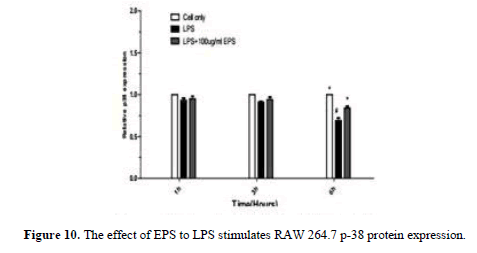
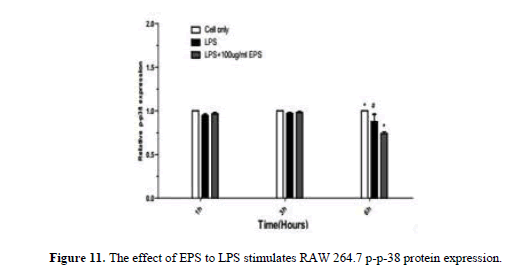
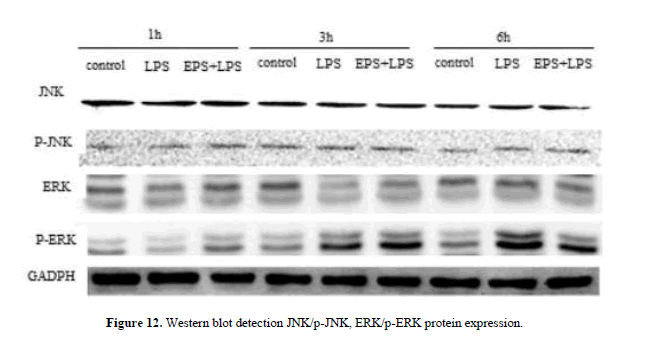
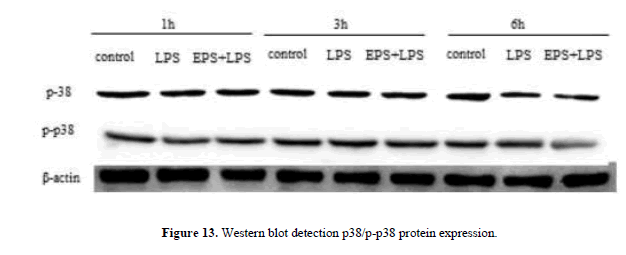
After LPS stimulates RAW264.7, JNK, p-JNK, ERK, p-ERK, p38, p-p38 protein expression increase by LPS group. JNK, p38, p-p38 protein expression increase at 1 h and 3 h,JNK, ERK, p-ERK, p-p38 protein expression significantly lower by EPS group than LPS group at 6 h (P<0.05) p38 protein expression significantly higher than LPS group (P<0.05) (Figures 6-11).
When the LPS stimulated RAW264.7, the expression of JNK, ERK, and p38 protein decreased significantly with the extended duration at 1 h, 3 h and 6 h compared with LPS group, the expression of p-JNK, p-ERK, p-p38 protein was significantly increased. The expression levels of JNK, ERK and p38 were higher by EPS group than that in LPS group, the expression of p-JNK, p-ERK, p-p38 protein was significantly reduced, and the changes of protein at 3 h and 6 h were obvious. EPS inhibition of inflammatory response was associated with activation of MAPK signaling pathway (Figures 12 and 13).
Discussion
When the LPS stimulated cell, IkB was activated and resulted in the phosphorylation of IkB protein. The IkB protein degradation, the NF-kB was released in the complex and then transferred to the nucleus to facilitate transcription. This test checked the contents of NF-kBp65, NF-kB, p-p65, IκB-α, p-IκB-α protein in the RAW264.7 nucleus, and the results showed When the LPS stimulated RAW264.7, the NF-κB, p-65 and p-IκB-α protein expression elevated. But the IκB-α protein expression decreased. At the same time EPS could inhibit NF- Kb protein expression elevated and IκB-α protein expression decreased. The effect of inhibition the NF-kB protein expression increased gradually with the extension of time. The activation of NF-kB is an important pathway for inflammatory lesions. Therefore, the effect of echinacea in inhibiting inflammatory response may be effective in inhibiting inflammatory response.
As an important proinflammatory cytokine, TNF-α is able to increase the generation of some other inflammation-related cytokines, such as IL-6 IL-1β and interferon-γ (Butler et al., 1997). As a principal component of the outer membrane of Gram-negative bacteria, LPS can enter the blood stream and conduce to a variety of inflammatory dysfunction which may ultimately lead to death (Wang J et al. 2014). LPS markedly decreased the IB-protein level by increasing its degradation and phosphorylation in murine microglial cells (Oh et al. 2013). IL-10 and dexamethasone have been shown to have anti-inflammatory effects through increasing the mRNA expression of NFBIA and inhibiting NF-kB activation (Ing et al. 2014). Inflammation is a major pathway involved in ALI induced by LPS (Kim et al. 2015). TLR4 is the major receptor through which LPS activates the inflammatory pathway (Zhang et al. 2015). Previous studies have shown increased mRNA expression levels of TLR4 after injection of LPS in rats (Wu et al. 2012; Cao et al. 2015). Activation of NF-kB is increased during inflammation and it is responsible for the expression of different inflammatory mediators induced by LPS (Lappas 2013). Since NFBIA is a major regulator of NF-kB activation (Nelson et al. 2004).
Activation of AMPK is known to exert an anti-inflammatory effect (Tang, J, et al. 2009; Ducommun, S, et al. 2014). LPS is known to induce ROS production, which plays an important role in subsequent NF- kB activation and TNF-α production (Zhao, Y. et al. 2004; Yuan, H. et al. 2009). A recent study by Jeon et al. (Jeon, S.M. et al. 2012) has established the anti-oxidant function of AMPK activation. Activated AMPK phosphorylates and inhibits its downstream target ACC, thus decreasing nicotinamide adenine dinucleotide phosphate (NADPH) consumption in fatty-acid synthesis and increasing NADPH generation by means of fatty-acid oxidation (Jeon, S.M. et al. 2012).
Conclusion
AMPK acts as a sensor of cellular energy status capable of regulating vital metabolic pathways in the cell (Hardie, D.G. et al. 2012). AMPK is activated by conditions that increase the AMP/ATP ratio (Hardie, D.G. et al. 2012). Meanwhile, metformin attenuates the cytokine-induced expression of pro-inflammatory and adhesion molecule genes by activation of AMPK and inhibiting NF-κB activation (Ducommun, S et al. 2014). Recent studies have shed lights on how AMPK activation attenuates oxidative stress. Activated AMPK could promote NADPH synthesis while limiting its consumption (Jeon, S.M. et al. 2012). Our recent study showed that AMPK activation is involved in perifosine’s inhibitory effect on TNF-α production. EPS inhibit inflammation may be associated with activation of MAPK signal pathway. When LPS stimulated RAW264.7, MAPK Cascade activation, which can be shown to be stage by stage phosphorylation, promoted cell division, proliferation, etc., thereby reduced the inflammatory injury of LPS to cells. To sum up, the damage of alveolar macrophages was closely related to the immune response mediated by the activation of MAPK signaling pathway in cells. EPS suppression of inflammation may be associated with activation of MAPK signaling pathways.
Acknowledgments
Research supported by the Project of National Major Basic Dairy Research “973” Plan (#2016CB100803) and by the grant of Ministry of Agriculture (#2016-G35).
About the Authors
Corresponding Author
Q.D. Jiang
Key Laboratory of Animal Biochemistry and Nutrition, Ministry of Agriculture, Henan University of Animal Husbandry and Economy, Zhengzhou, Henan, China
- Email:
- jiangqd2000@126.com
References
- Bao S, Zou Y, Wang B, Li Y, et al. (2015). Ginsenoside Rg1 improves lipopolysaccharide-induced acute lung injury by inhibiting inflammatory responses and modulating infiltration of M2 macrophages. Int Immunophar-macol. 28: 429- 434. https://doi.org/10.1016/j.intimp.2015.06.022
- Butler DM, Malfait AM, Mason LJ, Warden PJ, et al. (1997). DBA/1 mice expressing the human TNF-a transgene develop a severe, erosive arthritis: Characterization of the cytokine cascade and cellular composition. J. Immunol. 159: 2867-2876.
- Cao Y, Hong HH, Yang JC, Zhao W, et al. (2015). Effect of qidonghuoxue decoction on inflammatory factors and TLR4 mRNA Expression in acute lung injury rats. 35:438-442.PMID:26043567.
- Ducommun S, Ford RJ, Bultot L, Deak M, et al. (2013) Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am. J. Physiol. Endocrinol. Metab. 30688-696. https://doi.org/10.1152/ajpendo.00672.2013
- Hardie DG, Ross FA, Hawley SA (2012). AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell. Biol. 13, 251-262. https://doi.org/10.1038/nrm3311
- Hagemann T, Balkwill F, Lawrence T (2007). Inflammation and cancer: A double-edged sword. Cancer cell. 12: 300-301. https://doi.org/10.1016/j.ccr.2007.10.005
- Ing NH, Forrest DW, Riggs PK, Loux S, et al. (2014). Dexamethasone acutely down-regulates genes involved in steroidogenesis in stallion testes. J. Steroid Biochem. Mol. Biol.143:451-459. https://doi.org/10.1016/j.jsbmb.2014.07.003
- Jeon SM, Chandel NS, Hay N (2012). AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 485: 661-665. https://doi.org/10.1038/nature11066
- Kim KY, Lee HS, Seol GH (2015) Eucalyptol suppresses matrixmetal oproteinase-9 expression through an extracellular signal-regulated kinase-dependent nuclear factor-kappa B pathway to exert anti-inflammatory effects in an acute lung inflammation model. J. Pharm. Pharmacol.67:1066-1074. https://doi.org/10.1111/jphp.12407
- Krishnamoorthy S, Honn KV (2006). Inflammation and disease progression. Cancer Metastasis Rev. 25:481-491.https://doi.org/10.1007/s10555-006-9016-0
- Lappas M (2013). NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kB. Biol. Reprod. 89: 14. https://doi.org/10.1095/biolreprod.113.110056
- Li Y, Wu Q, Deng Y, Lv H, et al. (2015). D (-)-Salicin inhibits the LPS-induced inflammation in RAW264.7 cells and mouse models. Int Immuno Pharmacol. 26: 286-294. https://doi.org/10.1016/j.intimp.2015.04.016
- Lou T, Jiang W, Xu D, Chen T (2015). Inhibitory Effects of Polydatin on Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflammation 38:1213-1220. https://doi.org/10.1007/s10753-014-0087-8
- Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, et al. (2004). Oscillations in NF-kB signaling control the dynamics of gene expression. Science. 306:704-708. https://doi.org/10.1126/science.1099962
- Oh WJ, Jung U, Eom HS, Shin HJ (2013). Inhibition of lipopolysaccharide-induced proinflammatory responses by Buddleja officinalis extract in BV-2 microglial cells via negative regulation of NF-kB and ERK1/2 signaling. Molecules.18:9195-9206.
- Tang J, Feng Y, Tsao S, Wang N (2009). Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J. Ethnopharmacol. 126:5-17. https://doi.org/10.1016/j.jep.2009.08.009
- Tao W, Su Q, Wang H, Guo S, et al. (2015) Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int Immunopharmacol. 27: 138-147. https://doi.org/10.1016/j.intimp.2015.05.005
- Wei D, Huang Z (2014). Anti-inflammatory effects of triptolide in LPS-induced acute lung injury in mice. Inflammation. 37:1307-1316. https://doi.org/10.1007/s10753-014-9858-5
- Wang J, Liu YT, Xiao L, Zhu L (2014). Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-kB Pathway. Inflammation. 37: 2085-2090. https://doi.org/10.1007/s10753-014-9942-x
- Wu F, Liu Y, Lv X, Miao X, et al. (2012) Small interference RNA targeting TLR4 gene effectively attenuates pulmonary inflammation in a rat model. J. Biomed. Biotechnol. 406-435. https://doi.org/10.1155/2012/406435
- Yuan H, Perry CN, Huang C, Iwai-Kanai E, et al. (2009). LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cryoprotection. Am. J. Physiol. Heart Circ. Physiol. 296:470-479. https://doi.org/10.1152/ajpheart.01051.2008
- Zhang Y, Zhang R, Qiao S, Fan J (2015) Resolvin D2 recovers neural injury by suppressing inflammatory mediator’s expression in lipopolysaccharide induced Parkinson’s disease rat model. Biochem. Biophys. Res. Commun. 460:799-805. https://doi.org/10.1016/j.bbrc.2015.03.109
- Zhao Y, Joshi-Barve S, Barve S, Chen LH (2004). Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-κB activation. J. Am. Coll. Nutr. 23:71-78. https://doi.org/10.1080/07315724.2004.10719345
Keywords:
Download:
Full PDF- Share This