Synonymous Variant of ACE Gene (rs4343) is Coupled with Early Age at Onset and Diminished Diabetic Duration in South Indian Diabetic Nephropathy Patients
Received: March 22, 2019
Accepted: March 26, 2019
Published: May 05, 2019
Genet.Mol.Res. 19(5):
Keywords
ACE rs4343 polymorphism; Diabetic Nephropathy; Age at onset and Disease duration of diabetes.
Introduction
Discerning the biological role of key bio-molecules play an important role in understanding the pathophysiology of numerous human diseases including type-2 diabetes (T2DM) which pave the path for their better management. As biochemical reactions are mediated by enzymes, variations in their genes may pose an advantage or disadvantage to the individuals carrying them towards complex diseases. One such gene Angiotensin Converting Enzyme (ACE) that was extensively studied in relation to variety of diseases impacted heavily in the management of several ACE dependent diseases (Sayed-Tabatabaei FA et al., 2006). Diabetic nephropathy (T2DN) a major micro vascular complication of T2DM is the largest single cause of end stage renal failure and abnormally active Renin-Angiotensin System (RAS) is shown to influence the development of T2DN. The inter-individual variation with respect to ACE levels, a crucial enzyme that converts angiotensin I to angiotensin II in RAS pathway partly reliant on the genetic variants of ACE, signifying its genotype may influence the progression and severity of diabetes and its complications (Chawla T et al., 2010). A large number of functional polymorphisms exist in this gene, rs4343 a synonymous mutation (Thr776Thr) in exon 17th is reported to alter mRNA folding that decrease its stability and transcription level (Keavney B et al., 1998; McKenzie CA et al., 2005). The HAPMAP revealed a varying frequency of minor allele of rs4343polymorphism in different populations, the least being in the African (0.3) and the highest in European population (0.54). The mutant minor allele is associated with increased serum ACE levels suggesting, high levels of ACE may independently or in interaction with other genes or environment may exert an effect that impact the genetic predisposition of an individual to the disease or course of the disease differently in different populations (Zhu X et al., 2001).
The present study sought to investigate the role of rs4343 polymorphism in the propensity, age at onset of diabetes and diabetic duration before the onset of nephropathy in south Indians. The importance of this study lies in the event of reduction in the age at onset of diabetes and diminishing duration of diabetes before the onset of T2DN globally as well as in our population.
Materials and Methods
Sampling and data collection
This case-control study comprises a total of 300 subjects of southern India. Patients diagnosed by an endocrinologist and nephrologists, of whom 100 were clinically identified type-2 diabetic nephropathy group (T2DN),100 type 2 diabetic patients without nephropathy (T2DM) and 100 healthy controls (HC) visiting Nizam’s institute of Medical sciences hospital (NIMS), Hyderabad, India. The present study was approved by Ethical committee of Maulana Azad National Urdu University, Hyderabad, India. Demographic, anthropometric details like age, gender, height, weight, waist and hip measurements, family history of the disease and duration of diabetes were recorded in a well-designed Performa. Informed consent was obtained prior to the sample collection; procedure and motive of the study were explained to the patients. Body mass index (BMI) and waist to hip ratio (W/H) was calculated as given elsewhere (Snehalatha C et al., 2003). Blood samples were obtained from the subjects for biochemical and molecular analysis. In order to the see the influence of rs4343 polymorphism on the age at onset of diabetes (AAODM) patients (T2DM+T2DN) were categorized into two age groups i.e. ≤ 45 years and > 45 years. Further stratification of DN patients were done based on duration of diabetes before onset of nephropathy into two groups 1) patients who develop nephropathy within 7 years (≤ 7) of diabetes 2) those who develop nephropathy after 7 years (>7) of diabetes duration.
Inclusion criteria
The inclusion criteria for the three different study groups were: a) T2DN patients: Individuals diagnosed with a urine albumin excretion of >300 mg/day on two different occasions in a span of 3-6 months without any clinical evidence of other kidney diseases and infectious condition. b) T2DM individuals without nephropathy: urinary albumin excretion of <30 mg/day (checked on three different occasions) and negative for dipstick urinary protein, longstanding diabetes for a period of 10 years with no history of hypertension prior to the development of diabetes. c) HC: unrelated healthy individuals with no history of diabetes, hypertension and renal disorders.
Biochemical analysis
Readings of Fasting and Post lunch Blood sugars (FBS, PLBS) were obtained from the medical records of the hospitals. Glycosylated hemoglobin (HbA1c) levels were estimated using a Nycocard reader (AXIS-SHIELD, Norway). The serum creatinine, albumin levels and lipid profile were measured by enzymatic methods in a semi-auto analyzer.
Molecular analysis
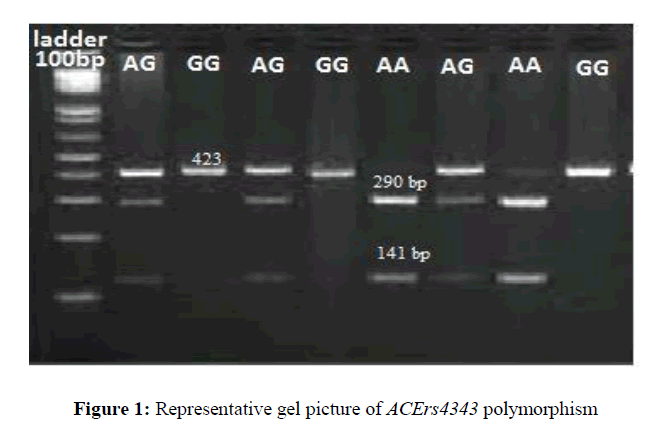
Genomic DNA was isolated from venous blood by using salting out method. The quantity and quality of DNA was assessed with spectrophotometer and gel electrophoresis. The genotyping for the ACE rs4343 (A2350G in exon 17) polymorphism was performed using PCR-Restriction Fragment Length Polymorphism (RFLP). The amplification reaction was carried out in a total volume of 10μl, using 100ng genomic DNA, 1μl of buffer, 0.3μl of dNTP, 0.2μl of each forward 5'AAGGAGAGGAGAGAGACTCA3' and reverse primer 5'TGTTGGCAGCAGGGACTCACC3' (Bioserve Biotechnologies, Hyderabad, India) 0.3μl of Taq polymerase (Labpro, Hyderabad, India). The PCR cycling conditions were: an initial denaturation at 95°C for 5 min followed by 30 cycles, including denaturation at 94°C for 30 sec, annealing at 58o C for 45 sec, and a final extension at 72°C for 5 min. The restriction enzyme BtsCI (Labpro, India) was used to distinguish the rs4343 genotyping. The digested PCR product showed the fragment size of 432 bp for mutant type (G) and 290 bp and 141 bp for wild type (A) On 2% agarose gel electrophoresis (Figure 1). Negative and positive controls were used in each genotyping run and few samples were randomly picked and re-genotyped to avoid mistyping of genotypes.
Statistical analysis
Descriptive statistics was done to calculate percentages, mean and standard deviation (SD). Student’s t-test was used to test for differences in various characteristics for continuous variables. The evaluation of data among the groups was performed by ANOVA (one-way ANOVA) test. Genotypic and allelic frequencies among study participants were analyzed with the Chi-square test and Hardy Weinberg Equilibrium (HWE) was calculated. Chi-square contingency tables were used to compare the allele and genotype frequencies between patients, controls and subgroups. The risk associated with genotypes was estimated using online odds ratio calculator at 95% confidence interval (CI). Relationship between the risk factors and T2DN was assessed through Multiple Logistic Regression (MLR) analysis. Data analysis was carried out by SPSS version 21 wherever required.
Results
Characteristics of the study group
A total of 300 subjects were recruited for the present study. The baseline anthropometric and clinical features of the study population are summarized in Table 1. Results are expressed as mean ± SD in healthy controls (HC), T2DM and T2DN. Two-way ANOVA was performed with respect to various anthropometric and clinical features to compare the means between HC, T2DM and T2DN groups and their mean ages at the time of sample collection was 62.67 ± 5.6, 59.37 ± 7.86 and 60.6 ± 7 years respectively.
| HC (n=100) X ± SD |
T2D (n=100) X ± SD |
T2DN (n=100) X ± SD |
p-value | ||
|---|---|---|---|---|---|
| Age ( years) | 62.67 ± 5.6 | 59.37 ± 7.86 | 60.6 ± 7 | 0.003 | |
| Males (%) | 50 (50) | 50 (50) | 50 (50) | - | |
| Females (%) | 50 (50) | 50 (50) | 50 (50) | - | |
| Mean of #AAODM | - | 49.5 ± 6.1 | 45.91 ± 5.53 | < 0.0001* | |
| BMI (Kg/m2) | 23.7 ± 3.51 | 24.95 ± 2.96 | 23.58 ± 3.4 | ||
| W/H ratio | 0.83 ± 0.08 | 0.93 ± 0.06 | 0.91 ± 0.03 | <0.001 | |
| HbA1c (%) | 4.9 ± 0.6 | 8.15 ± 1.62 | 8.76 ± 1.51 | <0.001 | |
| Serum creatinine (mg/dl) | 0.82 ± 0.19 | 1.14 ± 0.54 | 3.96 ± 2.38 | <0.001 | |
| Serum albumin (g/dl) | 4.2 ± 0.55 | 4.07 ± 0.61 | 3.03 ± 0.67 | <0.001 | |
| Total cholesterol (mg/dl) | 152.12 ± 31.21 | 178.72 ± 42.77 | 202.61 ± 49.97 | <0.001 | |
| High density lipoprotein (mg/dl) | 48.77 ± 5.34 | 42.68 ± 11.96 | 30.83 ± 11.09 | <0.001 | |
| Low density lipoprotein (md/dl) | 71.44 ± 17.64 | 109.58 ± 46.33 | 140.03 ± 55.77 | ||
| Very low density lipoprotein | 17.85 ± 6.43 | 28.94 ± 17.99 | 32.02 ± 9.44 | <0.001 | |
| Triglycerides (mg/dl) | 114.47 ± 34.72 | 171.1 ± 86.71 | 196.65 ± 61.33 | <0.001 | |
| TC/HDL ratio | 3.26 ± 0.75 | 4. ± 0.7 | 7.55 ± 3.41 | <0.001 |
One way anova analysis for all the variables (except mean of AAODM) was performed as a test of significance; *t-test p-value; #AAODM= age at onset of diabetes mellitus.
Table 1: Demographic and clinical characteristics of the study group.
Genotype association analysis
Table 2 represents the distribution of ACE rs4343 genotypes, allelic frequencies and their odds ratio among the studied population. The current analysis revealed that percentage distribution of GG genotypes was more in T2DN (39%) when compared to T2DM (20%) and HC (18%) subjects. The frequency of AG genotype was marginally high among T2DM (43%) than T2DN (39%) and HC (33%). Higher frequency of AA genotype was found among HC (49%) than T2DM (37%) and T2DN (22%) individuals. The G and A allele frequencies were 0.35, 0.65; 0.42, 0.58 and 0.59, 0.42 respectively in HC, T2DM and T2DN subjects. The ACE rs4343 genotype frequencies were found to be in Hardy Weinberg Equilibrium (HWE) among the T2DM group (χ2 = 1.31, p =0.25) whereas, a deviation of the genotype frequencies from HWE was observed in the HC and T2DN groups (χ2 = 7.28, p= 0.006; χ2 = 3.87, p =0.05 respectively). Overall analysis between patients and control group (T2DN+T2DM vs. HC) revealed that GG genotype to be imparting 2 fold increased risk toward diabetes (OR-1.91; 95% CI=1.05–3.45; p<0.05) whereas AA genotype was found to be protective (OR-0.43 95% CI=0.26–0.71; p <0.001). The subgroup analysis between HC and T2DM did not show any genotype dependence (p>0.05), while between HC and T2DN as well as between T2DM and T2DN groups replicated the results as that of overall patients and controls with varying folds and significance levels (p <0.05).
| Category (N) |
AA (%) | AG (%) | GG (%) | χ2 value (p-value) |
Allele frequency | Group Comparison |
OR (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| G | A | ||||||||
| Patients (DM+T2DN) (200) HC (100) |
59(29.5) 49(49) | 82(41.0) 33(33) | 59(29.5) 18(18) | 11.59 (0.003) |
0.50 0.35 | 0.50 0.65 | AA vs. AG + AA AG vs. AA + GG GG vs. AA + AG |
0.43 (0.26-0.71) 1.41 (0.85-2.33) 1.91 (1.05-3.45) |
0.001 0.2 0.03 |
| HC (100) DM(100) | 49(49) 37(37) | 33(33) 43(43) | 18(18) 20(20) | 3.09 (0.21) |
0.35 0.42 | 0.65 0.58 | AA vs. AG + AA AG vs. AA + GG GG vs. AA + AG |
0.61 (0.34 - 1.07) 1.53 (0.86 - 2.72) 0.13 (0.56 - 0.71) |
0.11 0.18 0.85 |
| HC (100) T2DN (100) | 49 (49) 22 (22) | 33(33) 39 (39) | 18(18) 39 (39) | 18.50 (<0.01) | 0.35 0.59 | 0.65 0.42 | AA vs. AG + AA AG vs. AA + GG GG vs. AA + AG |
0.33 (0.17 - 0.61) 1.29 (0.72 - 2.31) 2.91 (1.52 - 5.57) |
0.0003 0.46 0.001 |
| DM(100) T2DN (100) | 37(37) 22(22) | 43(43) 39(39) | 20(20) 39(39) | 10.13 (<0.01) |
0.42 0.59 | 0.58 0.42 | AA vs. AG + AA AG vs. AA + GG GG vs. AA + AG |
0.53 (0.28 – 1.0) 0.85 (0.48-1.59) 2.55 (1.35-4.81) |
0.06 0.66 0.005 |
H.W.E:HC-χ2= 7.28 (p 0.006), T2DM Patients-χ2=1.31 p:0.25, T2DN Patients –χ2= 3.87, p:0.05
Table 2: Distribution of genotype and allele frequency of ACE rs4343 gene polymorphism among patients and controls.
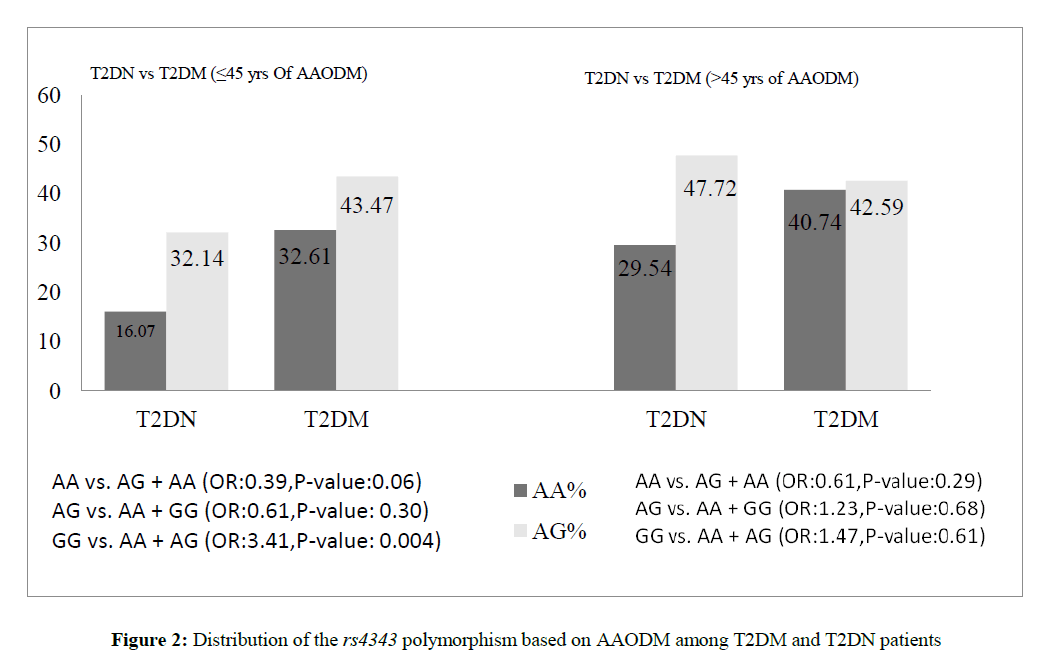
Figure 2 represent the ACE rs4343 genotype distribution in relation to AAODM among T2DM and T2DN patients. In ≤ 45 years category, both G allele and GG genotype were found to be elevated in T2DN and T2DM groups (0.68% vs. 0.46%; 51.78% vs. 23.91%). While, an enhanced frequency of A allele and AA genotype were observed in T2DM group compared to T2DN group (0.54% vs. 0.32%; 32.61%vs. 16.07%). No genotype variation was observed among the older age group i.e. >45 years (p>0.05).
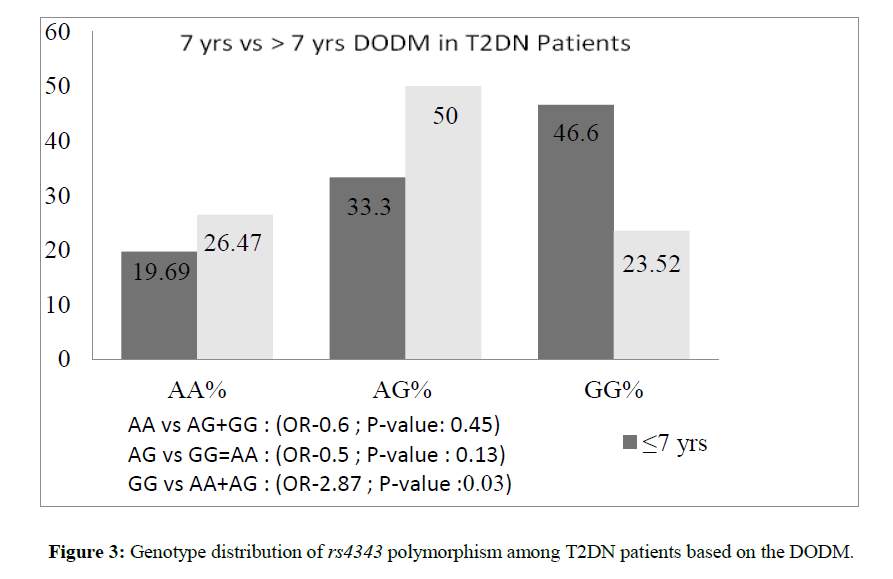
Figure 3 shows the genotype distribution of T2DN patients based on the DODM prior to the onset of nephropathy. There was a 3 fold increased risk for patients with GG genotype to develop nephropathy within ≤ 7 years of DODM and AA genotype seemed to have a protective effect, as patients with this genotype were represented more in the latter group.
Multiple Linear regression analysis was done aiming at identifying the independent risk factors for T2DN. Among T2DN vs. T2DM groups MLR revealed that BMI, W/H ratio, AAODM, DODM and ACE rs4343 GG genotype were associated with an enhanced risk for T2DN (Table 3).
| Variables | OR (95% CI) | p-value |
|---|---|---|
| Gender | 0.75 (0.35-1.6) | 0.46 |
| BMI | 1.65 (1.01-2.69) | 0.05* |
| W/H Ratio | 1.68 (0.45-3.76) | 0.04* |
| AAODM | 1.89 (0.86-1.99) | 0.02* |
| DODM | 1.11(1.08-1.24) | 0.01* |
| HbA1c | 2.09 (1.29 -5.33) | 0.99 |
| T.C. | 1.85 (0.68-5.02) | 0.22 |
| TC/HDL ratio | 2.56 (0.35-18.47) | 0.11 |
| T.G. | 1.19 (0.51-2.76) | 0.67 |
| GG genotype | 2.43 (1.08-5.47) | 0.03* |
*p<0.05
Table 3: Multiple Logistic Regression Analysis results of ACE rs4343 in T2DNvs. T2DM group.
Discussion
Asian Indians, an ethnically distinct population, lead the world in the number of people with type-2 diabetes (T2DM) and associated complication nephropathy (T2DN) which contributes both in terms of morbidity, mortality and socioeconomic loss due to End stage renal failure. T2DM is a complex disorder whose etiopathophysiology involves numerous genetic and environmental factors and their interactions (Wild S et al., 2004; De Fronzo RA et al., 2015; Lin YC et al., 2018). The efforts of scientists in understanding the implicated minor and major genes both in the genetic predisposition and progression of T2DM and its complications will aid to the better management of these conditions.
Based on the experimental and epidemiological data it is well established that activation of the RAS pathway plays an important role in elevating the micro and macro vascular problems in patients with T2DM (Gurley SB, Coffman TM 2007). ACE inhibitors have been reported to improve kidney, heart function, vascular compliance, endothelial derived nitric oxide production, vascular relaxation, plasma markers of inflammation, oxidative stress and thrombosis inT2DN individuals. The favorable effects of ACE inhibitors are due to the influence on hemodynamic as well as tissular effects of Angiotensin II (Cordonnier DJ et al., 2001; Samy I McFarlane et al., 2003).
Angiotensin converting enzyme is one of the crucial enzyme in RAS pathway. The inter-individual variation seen among the subjects with respect to ACE levels is dependent on the gene variants of ACE, suggesting its genotype may influence the severity and progression ofT2DM and T2DN (Rahimi Z et al., 2012). A large number of polymorphisms exist in ACE gene with functional significance. rs4343 polymorphism is one among them, a synonymous variant that is associated with increased ACE levels. Generally the synonymous variants alter the gene expression either through change of codon usage frequency, a change in mRNA secondary structure and stability or a change in normal splicing pattern (Chamary JV et al., 2006; Joanna L Parmley and Hurst 2007).
McKenzie et al., 2005 demonstrated G allele of rs4343 polymorphism leads to significant change in ACE expression by altering mRNA folding (McKenzie et al., 2005).
This genetic variant of ACE gene has not been sufficiently explored as well there are no studies pertaining to south Indian population (Table 4). Inflammation and oxidative stress (OS) coupled disorders are shown to be associated with elevated levels of Angiotensin II and diabetes is one among them with chronic low grade inflammation and increased OS. The primary objective of the current study was of three fold, to see whether this polymorphism has an impact on (i) the propensity of T2DM and T2DN. (ii) the age at onset ofT2DM and T2DN. (iii)The duration of diabetes (DODM) before the onset of nephropathy. is being clinically initiated.
| Authors | Year | Country / Population |
Disease |
|---|---|---|---|
| Parul Aggarwal | 2016 | N.India | Gestational diabetes |
| P.huo, D, et al | 2015 | N.Chinese | Diabetic Nephropathy |
| Cheema et al | 2013 | Asian Indians | Type 2 Diabetic |
| Ahluwalia TS, et.al. | 2009 | Asian Indian | Type 2 diabetic nephropathy. |
| Narita, et.al. | 2003 | Japanese | IgA nephropathy |
Table 4: rs4343 studies in different populations on related to Diabetes / Nephropathy.
In the present study significant augmented association of mutant G allele and GG genotype in T2DN, A allele and AA genotype in T2DM individuals suggest their predisposing and protective roles towards nephropathy. Our results are in conformity with Hua et al. (2015) who demonstrated a link between GG genotype of rs4343 polymorphism with T2DN compared to that of T2DM patients indicating that G allele imparts susceptibility towards nephropathy (Huo P et al., 2015). Su et al. (2012) reported an association of this polymorphism with chronic kidney disease in Chinese Han population (Su SL et al., 2012). Contrary to above reports, Ahluwalia et al. (2009) showed lack of association in North Indian diabetic nephropathy patients (Ahluwalia TS et al., 2009). At large this advocates ACE may be an important factor in contributing to kidney diseases through Ang II mediated vasoconstriction, inflammation, OS and fibrosis. Auxiliary investigations with regard to this variant of ACE gene in diverse populations are necessary, given the limitation of the present study by the sample size.
The second objective of the current study was to see the influence of rs4343 on the AAODM among the patient group. Generally early-onset diabetes is considered to be with an age cutoff of 30–45 years. Even though globally T2DM is considered as a disease allied to aging, the prevalence of adult early-onset type has increased and higher proportion of early-onset T2DM was reported in Asian countries with a 4-fold increase from 1997 to 2010 in China than in Western countries (BK Lee et al., 2016). In our study we observed that 40% of T2DM patients developed the disease with in ≤ 45 years (45 years mean age at onset) and 60% of patients after 45 yrs of age (52 years mean age at onset. Further, we found that Diabetic patients with ≤ 45 years of age and carrying two mutant alleles exhibited a threefold increased tendency towards nephropathy than the T2DM patients with other genotypes, suggesting the possible interaction of this genotype with lifestyle factors. Further studies dealing with lifestyle factors may help in understanding the modifying effect of this polymorphism on the age at onset of T2DM and T2DN.
The third objective of ours was to investigate the influence of rs4343 polymorphism on the DOD before the onset of nephropathy within the T2DN group. The observation of increased frequency of patients with the mutant homozygous genotype who developed T2DN ≤ 7 years of diabetic duration compared to those who developed nephropathy after 7 years of T2DM suggest that the high producing genotype may speed up the progression or reduce the time of progressing towards nephropathy. It is well known that overt nephropathy caused by glomerulosclerosis appears after 5 to 10 years in patients with T2DM and the chances of nephropathy increases with the duration of T2DM (Inassi J and Vijayalakshmy, 2013). Based on our results we conclude that rs4343 polymorphism has a role not only in the susceptibility but also in AAO and DOD of T2DM and T2DN.
T2DM and T2DN being a complex condition depends on multiple intrinsic and extrinsic factors. We sought to explore the independent effect of potential risk factors for T2DN using MLR which has reemphasized the role of the GG genotype, AAO and DOD in the occurrence of nephropathy. In addition BMI and W/H ratio also emerged out as contributing factors (Schuler R et al., 2017). A large study considering various anthropometric and lifestyle factors is warranted to substantiate these observations.
Conclusion
In conclusion, we found an association of ACE rs4343 polymorphism with both T2DM and T2DN in our population. Further we suggest that this polymorphism influences not only the genetic predisposition to diabetes and diabetic kidney disease, but also the age at onset and duration of disease before the setting in of the complications. The limitation of the study is small sample size and lack of information of ACE levels. Large studies considering age at onset, duration of disease and various lifestyle factors among different ethnic groups substantiate the results and will be valuable in the management or therapeutic intervention through ACE inhibiters.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgment
This study was supported by UGC Non-Net Fellowship and Minor Research Project from Maulana Azad National Urdu University, Hyderabad.
About the Authors
Corresponding Author
Parveen Jahan
Department of Zoology, Moulana Azad National Urdu University, Gachibowli, Hyderabad, India
- Email:
- dr.pjahan@gmail.com
References
- Balnee BS, Kohli HS, Sharma R, Shah VN, et al. (2013) RAS Gene Polymorphisms and Renal Responsiveness to RAS Inhibition Therapy in Type 2 Diabetic Asian Indians. J Pharmacogenom Pharmacoproteomics 4 https://doi.org/10.4172/2153-0645.1000114
- Lee BK, Kim SW, Choi D, Cho EH (2016) Comparison of Age of Onset and Frequency of Diabetic Complications in the Very Elderly Patients with Type 2 Diabetes. Endocrinol Metab 31: 416. https://doi.org/10.3803/enm.2016.31.3.416
- Chamary JV, Parmley JL, Hurst LD (2006) Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat Rev Genet 7: 98-108. https://doi.org/10.1038/nrg1770
- Cordonnier DJ, Zaoui P, Halimi S (2001) Role of ACE inhibitors in patients with diabetes mellitus. Drugs 61:1883-1892. https://doi.org/10.2165/00003495-200161130-00001
- Sayed-Tabatabaei FA, Oostra BA, Isaacs A, Van Duijn CM, et al. (2006) ACE polymorphisms.Cir Res 98: 1123-1133. https://doi.org/10.1161/01.res.0000223145.74217.e7
- Gurley SB1 and Coffman TM (2007) The renin-angiotensin system and diabetic nephropathy. Semin Nephrol Mar 27: 144-152. https://doi.org/10.1016/j.semnephrol.2007.01.009
- Narita I, Goto S, Saito N, J Song J, et al. (2003) Renoprotective efficacy of renin–angiotensin inhibitors in IgA nephropathy is influenced by ACE A2350G Polymorphism. J Med Genet 40: 130e-130. https://doi.org/10.1136/jmg.40.12.e130
- Inassi J and Vijayalakshmy (2013) Role of duration of diabetes in the development of nephropathy in type 2 diabetic patients. National Journal of Medical Research 3.
- Joanna L, Parmley K, Laurence Hurst D (2007) How do synonymous mutations affect fitness? Bioessays 29: 515-519. https://doi.org/10.1002/bies.20592
- Keavney B, McKenzie CA, Connell JM, Julier C, et al. (1998) Measured haplotype analysis of the angiotensin-converting enzyme gene. Human Molecular Genetics 7: 1745-1751. https://doi.org/10.1093/hmg/7.11.1745
- McKenzie CA, Sinsheimer JS, Adeyemo AA, Cox RD, et al. (2005) SNP haplotypes in the angiotensin I-converting en¬zyme (ACE) gene: analysis of Nigerian family data using gamete competition models. Annals of Human Genetics 69: 227-232. https://doi.org/10.1046/j.1529-8817.2004.00142.x
- Aggarwal P, Agarwal N, Das N, Dalal K (2016) Association of polymorphisms in angiotensin-converting enzyme gene with gestational diabetes mellitus in Indian Women. Int J Appl Basic Med Res 6: 31-37. https://doi.org/10.4103/2229-516x.174006
- Huo P, Zhang D, Guan X, Mei Y, et al. (2015) Association between genetic polymorphisms of ACE &eNOS and diabetic nephropathy. Mol Biol Rep 42: 27-33. https://doi.org/10.1007/s11033-014-3736-y
- Poornima S, Subramanyam K, Khan IA, Hasan Q (2014) The insertion and deletion (I28005D) polymorphism of the angiotensin I converting enzyme gene is a risk factor for osteoarthritis in an Asian Indian population. J Renin Angiotensin Aldosterone Syst 16: 1281-1287. https://doi.org/10.1177/1470320314547403
- Schuler R, Martin Osterhoff A, TuridFrahnow P (2017) High-saturated-fat diet increases circulating angiotensin-converting enzyme, which is enhanced by the rs4343 polymorphism defining persons at risk of nutrient-dependent increases of blood pressure. J Am Heart Assoc 6: e004465. https://doi.org/10.1161/jaha.116.004465
- De Fronzo RA, Ferrannini E, Groop L, Henry RR, et al. (2015) Type 2 diabetes mellitus. Nature Reviews. Disease Primers 1: 15019. https://doi.org/10.1038/nrdp.2015.19
- Mc Farlane IS, Kumar A, James Sowers R (2003) Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol 91: 30-37. https://doi.org/10.1016/s0002-9149(03)00432-6
- Snehalatha C, Vishwanathan V, Ramachandran A (2003) Cut off values for normal anthropometric variables in Asian Indian Adult. Diabetes care 26: 1380-1384. https://doi.org/10.2337/diacare.26.5.1380
- Su SL, Lu KC, Lin YF, Hsu YJ, et al. (2012) Gene polymorphisms of angiotensin-converting enzyme and angiotensin II type 1 receptor among chronic kidney disease patients in a Chinese population. J Renin Angiotensin Aldosterone Syst 13: 148-154. https://doi.org/10.1177/1470320311430989
- Chawla T, Sharma D, Singh A (2010) Role of the renin angiotensin system in diabetic nephropathy. World J Diabetes 1: 141-145. https://doi.org/10.4239/wjd.v1.i5.141
- Ahluwalia TS, Ahuja M, Rai TS, Kohli HS, et al. (2009) ACE Variants Interact with the RAS Pathway to Confer Risk and Protection against Type 2 Diabetic Nephropathy. DNA Cell Biol 28: 141-150. https://doi.org/10.1089/dna.2008.0810
- Wild S, Roglic G, Green A, Sicree R, et al. (2004) Global prevalence of diabetes, estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053. https://doi.org/10.2337/diacare.27.5.1047
- Zhu X, Bouzekri N, Southam L, Richard S, et al. (2001) Linkage and association analysis of angiotensin I−converting enzyme (ACE)−Gene polymorphisms with ACE concentration and Blood Pressure. Am J Hum Genet 68: 1139-1148. https://doi.org/10.1086/320104
- Zuben Sauna E, Kimchi-Sarfaty C, Suresh Ambudkar V, Gottesman MM (2007) The sounds of silence: synonymous mutations affect. Function. Pharmacogenomics 8: 527-532. https://doi.org/10.2217/14622416.8.6.527
- Rahimi Z (2012) ACE insertion/deletion (I/D) polymorphism and diabetic nephropathy. J Nephropathol 1: 143-151. https://doi.org/10.5812/nephropathol.8109
Keywords:
Download:
Full PDF- Share This