Null polymorphisms in GSTT1 and GSTM1 genes and their associations with smoking and cervical cancer
Received: January 31, 2018
Accepted: May 10, 2018
Published: May 15, 2018
Genet.Mol.Res. 17(2): http://dx.doi.org/gmr16039918
DOI: http://dx.doi.org/10.4238/gmr16039918
Abstract
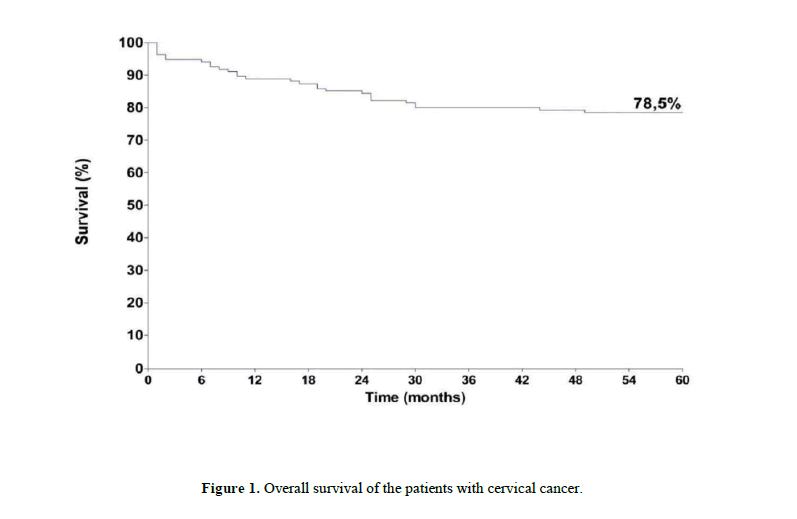
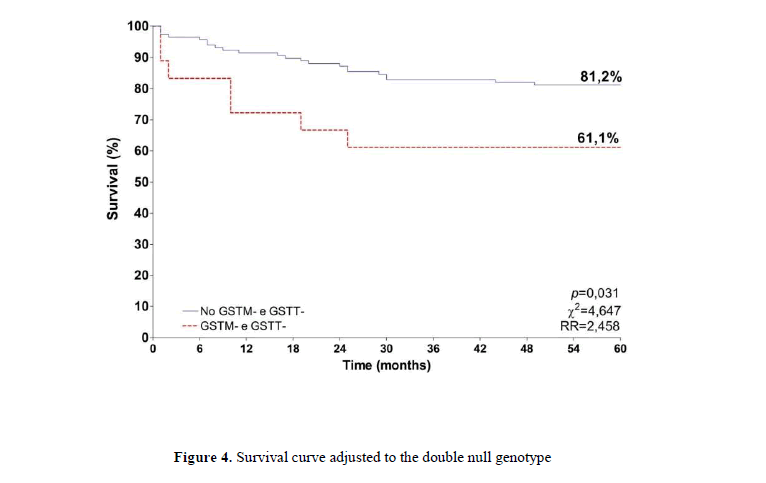
Human Papillomavirus (HPV) infection is the main risk factor for cervical cancer. However, other risk factors include smoking and genetic susceptibility. Glutathione-S-Transferases (GST) is enzymes involved in tobacco carcinogens metabolism and genes encoding these enzymes are highly polymorphic. The objective of this study was to compare the frequencies of GSTM1 and GSTT1 null polymorphisms in women with cervical cancer and in a control group, as well as the possible associations between such polymorphisms, cigarette smoking and the prognosis of cervical cancer. The series comprised 135 cervical cancer patients and 100 women without cancer. Genotypes were investigated by using polymerase chain reaction (PCR). The results were compared using the Chi-square test or Fisher's exact test, and survival analysis by Kaplan-Meier test and Log-rank. Among the cases, the frequency of GSTM1 gene null polymorphism was 22.2%, and for the GSTT1 gene it was 48.5%. Among the controls, the frequency of the GSTM1 gene null polymorphism was 45.0%, as for the GSTT1 it was 56.0%. Important association was demonstrated between smoking and cervical cancer (p=0.0062; OR=2.16). Differently from GSTM1, GSTT1 null polymorphism was not associated with cervical cancer risk in this study. GSTT1 null genotype was significantly associated to a worse prognosis. Overall survival rate for the cervical cancer group was 78.5% and, when stratified by genotypes, survival was higher in patients with positive genotypes, indicating a higher risk of death for those presenting dual nullity (p=0.031; RR=2.458).
Introduction
The main risk factor for cervical cancer is the infection with Human Papillomavirus (HPV) (Waggoner, 2003; Doorbar, 2012). HPV infection is necessary, but not enough for the development of cervical cancer. Other risk factors have become important such as viral genotype, persistence of HPV infection, genetic factors, immunological status and smoking (Campaner et al., 2011). Genetic susceptibility factors affecting the development of the cervical cancer have been studied in many populations (Li et al., 2013; Abbas et al., 2014; Roszak et al., 2014), but little is known about the interactions between genetic polymorphism and risk factors for cervical cancer, such as smoking (Roszak et al., 2014).
Chemical substances produced through tobacco burning and absorbed by the lungs can be found in the cervical mucus of smoker women, directly exposing the cervical cells to the tobacco carcinogens (Campaner, 2011). Smoking was reported as an important risk factor to cervical cancer. The time and intensity of the smoking habit are related to the development of cervical precursor lesions and invasive carcinoma, with smoking increasing the risk of neoplasia (Roura et al., 2013).
Tobacco contains many carcinogenic substances, such as nitrosamines and aromatic hydrocarbons, that interact with the genetic material of the cells resulting in DNA adducts, which in turn favor the occurrence of mutations that drive the carcinogenic process. Chemical substances present in tobacco are metabolized by enzymes from the P450 cytochrome family and glutathione-S-transferases (GSTT1 and GSTM1) (Leme et al., 2010).
Glutathione is an antioxidant tripeptide, comprising glycine, cysteine and glutamic acid. It is a cofactor in many enzymes and detoxification processes (Carreiro, 2011). Glutathione-S-transferases (GST) are a family of enzymes involved in the detoxification of xenobiotics, such as the polycyclic aromatic hydrocarbons present in cigarettes, hence protecting cells from deleterious and oxidative effects (Joseph et al, 2006; Singh et al, 2008; Leme et al., 2010). The homozygous deletion or null genotype of GSTM1 and GSTT1 results in the reduction or absence in the activities of these enzymes, with the possibility of increasing the susceptibility to cancer (Joseph et al, 2006; Singh et al, 2008; Leme et al., 2010). The genes that codify these enzymes are highly polymorphic and show great variability of expression levels and activity (Leme et al., 2010).
Brazilian population is extremely mixed and studies about the genetic factors, such as null polymorphism, in xenobiotic metabolic genes are still scarce. Despite the high potential of cervical cancer prevention, thousands of new cases are registered each year. Therefore, the investigation of factors associated to the development and to the prognostic of cervical cancer can improve prevention and treatment planning for such cancers.
Thus, the objective of this study was to evaluate the possible associations between the null polymorphisms of the GSTT1 and GSTM1 genes, smoking and the prognosis of cervical cancer patients.
Material and Methods
The current study was approved by the Ethics Committee in Human Research from the Association against Cancer in Goiás (CEP/AACG). The selection of the subjects with cervical cancer was achieved through active search in the files of the institutional Pathology Laboratory. Cervical cancer cases diagnosed between 2006 and 2007, were histopathologically confirmed and selected for the analysis of GSTT1 and GSTM1 null polymorphisms. Clinical and pathological data were collected from the medical files and included: histological type, histological grade, marital status, age at diagnosis, smoking and alcohol consumption history, menstrual status, type of treatment, clinical staging (extension of the lesion, lymph node metastasis and distant metastasis) and patient status in the last visit with a follow up of 60 months.
The control group included women without cervical cancer history paired by age group, who visited the clinical Laboratory of PUC - Goiás for routine exams. The group of women that participate in the study signed the Written Informed Consent, authorizing the collect of a blood sample for the molecular analysis of GSTT1 and GSTM1 null polymorphisms.
DNA extraction of the cervical tumor samples was performed by using the commercial Wizard Kit (Promega), according to the deparaphinization and extraction protocol. DNA extraction of peripheral blood samples of the control group was performed by the salting-out method (Sambrook, Fritsch and Maniatis, 1989).
Polymerase chain reaction (PCR) was used for the amplification of a 75 bp fragment to the GSTM1 gene and a 70 bp fragment for the GSTT1 gene, according to the protocol described by Halolu, 2013. The primers used for the amplification of the GSTM1 fragment were: Forward 5’ATGGTTTGCAGGAAACAAGG3’ and Reverse 5’CCTCCATAACACGTGAAGCA3’. The amplification of the GSTT1 gene employed: Forward 5’TTCCTGGGTGAGCCAGTATC3’ and Reverse 5’ACTGCAGGGTCACATCCAA3’ primers. All the reactions included positive and negative controls for the verification of the amplification efficiency. The amplification products were analyzed in 8% polyacrylamide gel and revealed by staining with silver nitrate.
Clinical and pathological data for both groups were compared in relation to the genetic polymorphisms. Chi-squared test or Fisher’s exact test were used, with a significance level in 5% (p < 0.05), using the GraphPad 4 Prisma software. Survival analysis used the Kaplan Meier and Log-rank method.
Results
The case group included 135 women with cervical cancer with confirmed histopathological diagnostic. The age of the group varied from 26 to 85 years and the average age was 49.6 years (±14.3). Cigarette smoking was described by 44.4% of the women and alcohol consumption by 9.6% of them. The majority of the women with cervical cancer were submitted to some type of surgery during treatment, including radical hysterectomy as the most frequent procedure (31.1%). As for the radiotherapy, the combination of external radiation + brachytherapy was applied to 40.0% of the women. Concerning histologic type, squamous cell carcinoma represented most of the cases (74.1%), followed by adenocarcinoma (17.0%). Concerning chemotherapy, it was used only in the metastatic cases. At the end of 60 months, death was registered for 23.0% of the group. The control group included 100 healthy women, with age varying from 24 to 86 years and the average of 54.3% years (±15.1). Cigarette smoking was reported by 27% of the women in the control group at a certain period of their lives. Out of these, 15.0% were still active smokers.
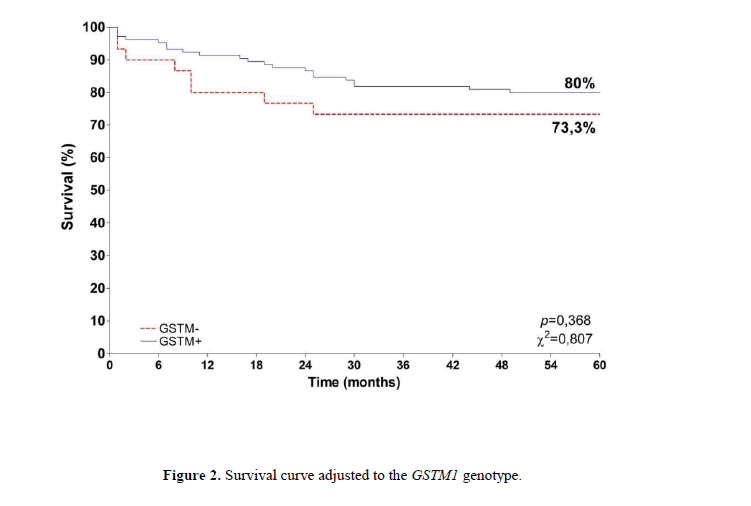
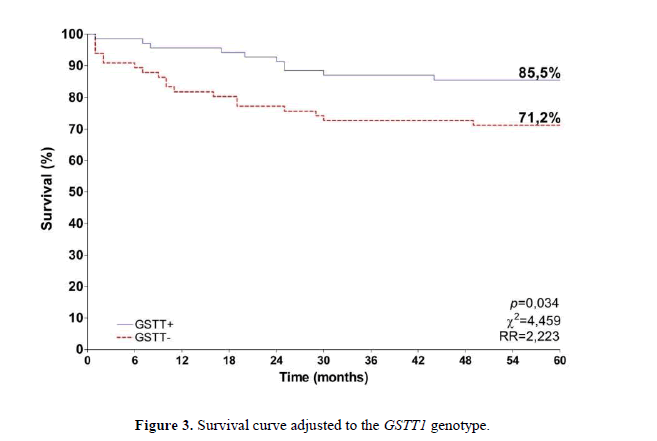
The frequency of GSTT1 and GSTM1 null polymorphisms is described in Table 1. GSTM1 null polymorphism was less frequent in cases (22.2%) than in controls (45.0%) (p=0.0016). An important relationship was observed between smoking habit and cervical cancer (p=0.0062), with a risk 2.16 times higher for smokers to develop cervical cancer (Table 2). The overall survival for the subjects with cervical cancer, at the end of 60 months, was 78.5% (Figure 1). When adjusted to GSTM1 genotype, the survival was 80.0% for the GSTM1 positive genotype and 73.3% for the GSTM1 null genotype carriers (Figure 2) (p=0.368). For GSTT1, the adjusted survival for the positive genotype carriers was 85.5% and 71.2% for the negative GSTT1 genotype carriers (Figure 3). A difference between the two groups was observed (p=0,034) and the risk for death was 2.2 times higher when the GSTT1 null genotype was detected.
When the double null genotypes were combined, the survival of patients carrying the double null polymorphism was 61.1%, while the survival for those with at least one of the alleles was 81.2% (Figure 4). A difference was described between the groups (p=0,031), with the risk of death 2.5 time higher for those with the double null genotypes.
| Gene | Null (%) genotype | Present (%) genotype | Null (%) genotype | Present (%) genotype | p | OR | ||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||
| GSTM1 GSTT1 GSTM1 and GSTT1 |
30 (22.2%) 66 (48.5%) 18 (13.3%) |
105 (77.8%) 69 (51.5%) 117 (86.7%) |
45 (45.0%) 56 (56.0%) 28 (28.0%) |
55 (55.0%) 44 (44.0%) 72 (72.0%) |
0.0016 0.2808 0.0051 |
0.4127 - 0.3956 |
||
Table 1. GSTT1 and GSTM1 null polymorphisms in the case and control groups.
| Variable | Cases (%) (n=135) | Controls (%) (n=100) | p | OR |
|---|---|---|---|---|
| Smokers | 60 (44.4%) | 27 (27.0%) | 0.0062 | 2.16 |
| Non-Smokers | 75 (55.6%) | 73 (73.0%) |
Table 2. Frequency of smoking habit between the case and control groups.
Discussion
According to the results obtained in this study a difference was observed in the frequency of GSTM1 and GSTT1 null polymorphisms between the group of patients with cervical cancer and controls. GSTM1 and GSTT1 null polymorphisms were both less frequent in cases than in controls. These results contradict the initial hypothesis of this study that the absence of these genes, which encode detoxification enzymes, would improve the process of carcinogenesis induced by HPV.
Many studies have investigated the relationship between the GSTM1 and GSTT1 null genotypes and different types of cancer, such as bladder, lung, prostate, kidneys, ovary, head and neck and others (Leme et al., 2010; Rodrigues et al, 2010; Halolu et al., 2013; Matic et al., 2013). However, due to the well-established relationship between cervical cancer and HPV, studies investigating these polymorphisms in cervical cancer are still scarce and differences between populations have been described. After reporting the presence of carcinogens derived from polycyclic aromatic hydrocarbons in the cervical mucus (Campaner et al., 2007; Kiran et al., 2010), the interest in the detoxifying enzymes started to increase in respect to cervical cancer. In Brazil only one published study investigated the frequency of GSTM1 and GSTT1 null polymorphisms in cervical adenocarcinomas and in controls without cancer (Carvalho et al., 2008). Thus, in Brazil, our study is the first investigating the frequency of the GSTM1 and GSTT1 null polymorphisms in cervical cancer including different histological types, such as squamous cell carcinomas, adenocarcinomas and adenosquamous carcinomas, compared to healthy controls. Brazilian studies about this topic investigated only the frequency of the polymorphism in the general population or the frequency of null genotypes in other type of cancers (Guembarovski and Cólus, 2001; Gatás et al., 2004; Leme et al., 2010; Olivera et al., 2010).
The relationship between the null polymorphisms of GSTM1 and GSTT1 with cervical cancer is controversial. Studies performed in different countries such as India (Joseph et al, 2006), Italy (Palma et al, 2010) and Kasakhstan (Djangusurova et al., 2013), considered the GSTM1 and GSTT1 null polymorphism as a risk factor to cervical cancer. On the other hand, studies developed in countries such as Thailand (Ishida et al, 2009), Turkey (Kiran et al, 2010) and Serbia (Stosic et al., 2014), did not considered GSTM1 and GSTT1 null polymorphism as a risk factor for cervical cancer. In a study developed in Colombia (Sierra-Torres et al, 2006), the GSTM1 and GSTT1 null polymorphisms didn´t affect the risk for high grade intraepithelial lesions, suggesting other factors as more important for cancer evolution, such as high-grade infection by HPV, exposition to mutagenic compounds and polymorphisms in other metabolism genes, such as the CYP2E1. A recent study developed in Thailand concluded that the deletion of GSTM1 is not a risk factor for cervical cancer. This study seems to present higher accuracy, since not only the null genotype for GSTM1 was studied but also the heterozygous variables for this gene (Natphopsuk et al., 2015).
A meta-analysis investigated this association in the world's population and found that GSTM1 null genotype confers increased risk for cervical cancer in non-Chinese population, while GSTT1 null polymorphism showed no association with cervical cancer (Economopoulos et al., 2010). Another more recent meta-analysis revised and updated the data of the Chinese population and found a significant association between the GSTM1 null polymorphisms and cervical cancer in that population (Sun and Song, 2016). The disagreement between the published results can be explained mainly by the different frequencies of these polymorphism in different populations, which can vary from 26.0% in Africans to 53.1% in Caucasians (Natphopsuk et al., 2015). Besides that, other factors can be associated to cancer development, such as lifestyle and exposition to different environmental xenobiotic. Important prognostic factors such as lymph node involvement, extension of the lesion and the number of deaths were less frequent in GSTM1 positive genotype carriers (data not shown). As for the deaths, it was observed that the majority of the living patients, at the end of 60 months (68.9%), showed a functional genotype; it means that some of the enzymatic activity might be retained in these patients, compared to those with double null genotypes. This data is reflected in the survival of the patients which was higher in all the situations where at least one positive genotype was observed, indicating a higher death risk in the cases with the double null genotype (p=0.031; RR=2.458).
One study evaluated cervical cancer survival associated with the GSTT1 and GSTM1 polymorphisms. In that study, the double null GSTM1 and GSTT1 genotype was associated to a higher survival (Abbas et al, 2015). It´s important to highlight that all the patients in the study were in advanced stages of the disease, and that all them went through treatment with cisplatin, a drug which is also metabolized by the GST enzymes. Therefore, the controversy between the results can be explained by the specificity of the group evaluated. It is known that the overexpression of GSTM and GSTT in tumor cells can determine the resistance to the treatment with cisplatin or derivative compounds and, in this case, the genetic null polymorphism would favor these subjects (Abbas et al., 2015).
Conclusion
Concerning the smoking habit, the well-established relationship (Philips and Venitt, 2012; Sobus and Warren, 2014; Wei et al., 2014) between tobacco and the risk for cervical cancer was also confirmed in our study (p=0.0062; OR=2.163). However, significant difference in survival was not observed between smokers and non-smoker (p=0.1845). Some limitations for this study included the size of the samples analyzed, the lack of information in the patient’s files and the molecular techniques employed for the polymorphism analysis. Concerning the size of the sample analyzed, despite the effort, it wasn´t possible to include all the population samples initially expected, resulting, therefore, in a sampling by convenience. As for the molecular technique, the conventional PCR does not allow the differentiation between homozygous and heterozygous genotypes, limiting the results to the null or present genotype. Through the results found, it’s concluded that GSTM1 null genotype was associated with cervical cancer in this study, and that GSTT1 positive genotype was significantly associated to a better prognosis in patients with cervical cancer. Further studies with cervical cancer and genes related to the metabolism of xenobiotic must include the analysis of other genes that act synergistically in the metabolism and elimination of toxic and mutagenic compounds. The inclusion of the genes from the P450 cytochrome family and the more detailed study of the GST genes, including the heterozygous genotypes, could increase the elucidation of the factors involved in cervical cancer development and xenobiotic metabolism, besides HPV.
Acknowledgments
FAPEG – Foundation for Research Support of the State of Goiás.
Clinical Laboratory of the Pontifical Catholic University of Goiás.
About the Authors
Corresponding Author
Ana Lúcia Munaro Tacca
Graduate Program in Environmental Sciences and Health, Pontifical Catholic University of Goiás, Brazil
- Email:
- verasaddi@gmail.com
References
- Abbas M et al. (2014). Association of CYP1A1 gene variants rs4646903 (T>C) and rs1048943 (A>G) with cervical cancer in a North Indian population. Eur J Obstet Gynecol Reprod Biol. 176: 68-74. https://doi.org/10.1016/j.ejogrb.2014.02.036
- Abbas M et al. (2015). Glutathione S-transferase gene polymorphisms and treatment outcome in cervical cancer patients under concomitant chemoradiation. PLOS One. 10 (11): e0142501. https://doi.org/10.1371/journal.pone.0142501
- Campaner, AB et al. (2011). Importância do tabagismo na carcinogênese do colo uterino. Femina. 35: 11.
- Carreiro DM. (2011). Terapia Nutricional no Estresse Oxidativo. In: SILVA, S M C S and MURA, J D P. Tratado de Alimentação. Nutrição e Dietoterapia. 2 ed. Roca.
- Carvalho CR et al. (2008). Polymorphisms of p53, GSTM1 and GSTT1, and HPV in uterine cervix adenocarcinoma. Eur J Gynaecol Oncol.29(6): 590-3.
- Djansugurova LB et al. (2013). The determination of genetic markers of age-related cancer pathologies in populations from Kazakhstan. Frontiers in Genetics. (4): article 70. https://doi.org/10.3389/fgene.2013.00070
- Doorbar J. (2012). The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 30: 55-70. https://doi.org/10.1016/j.vaccine.2012.06.083
- Economopoulos, K P et al. (2010). GSTM1 polymorphism, GSTT1 polymorphism, and cervical cancer risk: A meta-analysis. International Journal of Gynecological Cancer. 20: 9.
- Gattás GIF. et al. (2004). Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Brazilian Journal of Medical and Biological Research. 37:451-458. https://doi.org/10.1590/s0100-879x2004000400002
- Guembarovski RL and Cólus IMS. (2001). Glutathione S-transferase M1 (GSTM1): Ethnic distribuition and relation with cancer. Semina: Ci. Biol. Saúde. 22: 3 - 9.
- Haholu A et al. (2013). Is There any association of Glutathione -S-Transferase T1 (GSTT1) and Glutathione-S-transferase M1 (GSTM1) gene polymorphism with gastric cancers? Pol J Pathol. 64 (4): 247-252. https://doi.org/10.5114/pjp.2013.39332
- Ishida W et al. (2009). Glutathione S-Transferase (GSTM1 and GSTT1) polymorphisms in cervical cancer in Northeastern Thailand. Asian Pacific J Cancer Prev. (10): 365-368.
- Joseph T et al. (2006). Germline genetic polymorphisms of CYP1A1, GSTM1 and GSTT1 genes in Indian cervical cancer: Associations with tumor progression, age and human papillomavirus infection. Gynecologic Oncology. 101 (3): 411-417. https://doi.org/10.1016/j.ygyno.2005.10.033
- Kiran B et al. (2010). GST (GSTM1, GSTT1and GSTP1) polymorphisms in the genetic susceptibility of Turkish patients to cervical cancer. J Gynecol Oncol. 21 (3):169-173. https://doi.org/10.3802/jgo.2010.21.3.169
- Leme CD et al. (2010). Análise dos genes GSTT e GSTM em pacientes com cancer de cabeça e pescoço. Rev Assoc Med Bras. 56(3): 299 – 303.
- Li Y et al. (2013). CYP1B1 C4326G polymorphism and susceptibility to cervical cancer in Chinese Han women. Tumour Biol. 34(6):3561-7.
- Matic M et al. (2013). GSTA1, GSTM1, GSTP1, and GSTT1 polymorphisms and susceptibility to smoking-related bladder cancer: A case-control study. Urologic Oncology. 31: 1184-1192. https://doi.org/10.1016/j.urolonc.2011.08.005
- Natphopsuk S et al. (2015). Lack of participation of the GSTM1 polymorphism in cervical cancer development in Northeast Thailand. Asian Pacific Journal of Cancer Prevention. 16 (5): 1935-1937. https://doi.org/10.7314/apjcp.2015.16.5.1935
- Oliveira AL et al. (2010). GSTT1, GSTM1, and GSTP1 polymorphisms and chemotherapy response in locally advanced breast cancer. Genetics and Molecular Research. 9 (2): 1045-1053. https://doi.org/10.4238/vol9-2gmr726
- Palma S. et al. (2010). Interaction between glutathione-S-transferase polymorphisms, smoking habit, and HPV infection in cervical cancer risk. J Cancer Res Clin Oncol. 136: 1101-1109. https://doi.org/10.1007/s00432-009-0757-3
- Phillips DH and Venitt S. (2012). DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Intenational Journal of Cancer. 131: 2733-2753. https://doi.org/10.1002/ijc.27827
- Rodrigues IL et al. (2010). Evaluation of the influence of polymorphic variants CYP1A1*2B, CYP1B1*2, CYP3A4*1B, GSTM1*0, and GSTT1*0 in prostate cancer. Urologic Oncology. 29(6): 654-663. https://doi.org/10.1016/j.urolonc.2010.01.009
- Roszak A. et al. (2014). CYP1A1 Ile462Val polymorphism as a risk factor in cervical cancer development in the Polish population. Mol Diagn Ther. 18(4): 445-450. https://doi.org/10.1007/s40291-014-0095-2
- Roura E et al. (2013). Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. Int J Cancer. 135(2) 453-466. https://doi.org/10.1002/ijc.28666
- Sambrook J, Fritsch, EF, Maniatis T. (1989). Molecular cloning: A laboratory manual. 2 ed., New York: Cold Spring Harbor Laboratory Press. https://doi.org/10.1002/abio.370050118
- Sierra-Torres CH et al. (2006). Exposure to wood smoke, HPV infection and genetic susceptibility for cervical neoplasia among women in Colombia. Environmental and Molecular Mutagenesis. 47 (7): 553-561. https://doi.org/10.1002/em.20228
- Singh Het al. (2008). Association of GSTM1, GSTT1 and GSTM3 gene polymorphisms and susceptibility to cervical cancer in a North Indian population. American Journal of Obstetrics and Gynecology. 198(3): 303. https://doi.org/10.1016/j.ajog.2007.09.046
- Sobus S and Warren GW. (2014). The biologic effects of cigarette smoke on cancer cells. Cancer. 120 (23): 3617-3626. https://doi.org/10.1002/cncr.28904
- Stosic I et al. (2014). Glutathione S-transferase T1 and M1 polymorphisms and risk of uterine cervical lesions in women from central Serbia. Asian Pacific Jounal of Cancer Prevention. 15 (7): 3201-3205. https://doi.org/10.7314/apjcp.2014.15.7.3201
- Sun P and Song W Q. (2016). GSTM1 null genotype and susceptibility to cervical cancer in the Chinese population: An updated meta-analysis. Journal of Cancer Research and Therapeutics. 12 (2): 712-715. https://doi.org/10.4103/0973-1482.154004
- Waggoner, SE. (2003). Cervical câncer. The Lancet. 361.
- Wei L et al. (2014). Tobacco exposure results in increased E6 and E7 oncogene expression, DNA damage and mutation rates in cells maintaining episomal human papillomavirus 16 genomes. Carcinogenesis. 35(10): 2373-2381. https://doi.org/10.1093/carcin/bgu156
Keywords:
Download:
Full PDF- Share This