NNT and NNT-AS1 expression levels are not different between bipolar patients and healthy subjects
Received: October 25, 2018
Accepted: November 12, 2018
Published: January 05, 2019
Genet.Mol.Res. 18(1):
Introduction
Nicotinamide nucleotide transhydrogenase (NNT) participates in redox and energy interconnections. While it is encoded by nuclear genome, the protein is placed in the mitochondrial inner membrane (Hoek and Rydstrom, 1988). It links reduction of NADP⺠by NADH to proton translocation (Jackson et al., 2015). It can also bring forth the converse reaction to produce NADH and preserve mitochondrial membrane potential through proton deflating in some pathological situations (Ho et al., 2017). This enzyme also couples the mitochondrial respiration and H2O2 detoxification via the thioredoxin/peroxiredoxin coordination. Drug-induced suppression of Nnt in brain mitochondria considerably diminished their capacity to absorb H2O2 in the presence of respiration substrates. Besides, Nnt silencing in N27 dopaminergic cells has changed basal, extra, and greatest mitochondrial oxygen absorption rates and made these cell vulnerable to constant upsurges in H2O2 and cell death after exposure to subtoxic concentrations of paraquat. The role of Nnt in interrelating metabolic and H2O2 antioxidant systems in brain mitochondria potentiated it as a therapeutic target to enhance the redox balance (Lopert and Patel, 2014). A long non-coding RNA (lncRNA) has been recognized to be transcribed from the opposite stand of NNT gene. NNT-antisense RNA1 (NNT-AS1) has been involved in the pathogenesis of several human cancer possibly though modulation of the mitogen-activated protein kinase signaling pathway (Wang et al., 2017). However, the role of this lncRNA in regulation of redox balance has not been clarified yet.
Based on the high susceptibility of brain to oxidative damage, this kind of cellular stress has an established role in the development of various psychiatric disorders. Several biochemical, genetic, pharmacological and preclinical evidences have highlighted implication of oxidative stress in the pathogenesis of bipolar disorder (Ng et al., 2008). In the current study, we evaluated peripheral expression of NNT and NNT-AS1 in bipolar patients and healthy subjects to explore the role of these transcripts in the pathogenesis of bipolar disorder.
Materials and Methods
Study participants
The current study was performed on blood samples obtained from 50 bipolar patients (Male/Female: 35/ 15) and 50 healthy subjects with the same sex ratio. The mean age ± standard deviation (SD) (age range) was 36.5 ± 9.32 (17-56) in patients and 33.62 ± 8.59 (14-52) in controls. Disease duration (mean ± SD (range)) age ant disease onset in bipolar patients were 3.86 ± 2.66 (1-14) and 32.64 ± 8.04 (15-48) respectively. Patients were included in the study if they met the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) criteria (Association, 2013). All patients were in euthymic phase. The following factors were regarded as exclusion criteria for control group: past history of psychiatric or neurodegenerative diseases, mental retardation, cancer or infection. Control subjects were non-smokers and were not on any treatment regimen. The study protocol was approved by ethical committee of Shahid Beheshti University of Medical Sciences. Written consent forms were obtained from all study participants.
Expression analysis
Three milliliters of peripheral blood samples was used for RNA extraction using Hybrid-R Blood RNA kit (GeneAll Biotech, Korea). Subsequently, cDNA was synthesized from RNA samples using PrimeScript 1st strand cDNA Synthesis kit (Clontech, Japan). Expression analysis was performed in Rotor Gene 6000 real-time PCR system using the primer and probes listed in Table 1. The HPRT1 gene was used as normalizer. The RealQ Plus Master Mix (Ampliqon, Denmark) was used for PCR. All reactions were performed in duplicate. Each run had a no template control to rule out possible contaminations. Thermal cycling conditions were a preliminary activation step for 5 minutes at 95ºC followed by 40 cycles at 95ºC for 20 seconds and 65ºC for 1 minute.
| Gene name | Primer and probe sequence | Primer and probe length | Product length |
|---|---|---|---|
| HPRT1 | F: AGCCTAAGATGAGAGTTC | 18 | 88 |
| R: CACAGAACTAGAACATTGATA | 21 | ||
| FAM -CATCTGGAGTCCTATTGACATCGC- TAMRA | 24 | ||
| NNT1 | F: AGCCACCTTCTGTGTTACTTGC | 22 | 137 |
| R: TAGCCCAGAGCTGCCATGAC | 20 | ||
| FAM-TCAACCGTCAGGCTGCCACTGCTG-TAMRA | 24 | ||
| NNT-AS1 | F: CTTCCACTCTCGGGGACAGG | 20 | 110 |
| R: GCACCAGGTTTGATTGACAAGG | 20 | ||
| FAM - TTGTCTCTGCCTCGGCCTGCGG -TAMRA | 20 |
Table 1. Sequences of primers and probes used for expression analysis.
Statistical Analysis
Data was analyzed using SPSS 22.0 software (IBM, Chicago, IL, USA). The significance of difference in genes expressions between patients and healthy subjects was assessed using independent T test. The correlation between gene expression and clinicopathologic data of patients were evaluated using regression model. P < 0.05 was considered as significant.
Results
Relative expressions of NNT and NNT-AS1 in bipolar patients compared with controls
Transcript levels of NNT and NNT-AS1 were not significantly different between bipolar patients and controls (Table 2).
| Genes | Parameters | Total patients (n=50) vs. total controls (n=50) | Male patients (n=35) vs. male controls (n=35) | Female patients (n=15) vs. female controls (n=15) |
|---|---|---|---|---|
| NNT1 | Expression ratio | 1.13 | 1.16 | 1.08 |
| p-value | 0.54 | 0.55 | 0.86 | |
| NNT1-AS | Expression ratio | 1.23 | 1.29 | 1.12 |
| p-value | 0.34 | 0.2 | 0.88 |
Table 2. Relative expressions of NNT and NNT-AS1 in bipolar patients compared with controls.
Correlation between expression of genes and clinical features
No significant correlation was found between relative expression of genes and age of study participants. Moreover, expression of either gene was correlated with age at disease onset or disease duration in cases (Table 3).
| Variables | NNT | NNT-AS1 | |||
|---|---|---|---|---|---|
| R | P value | R | P value | ||
| Case | Age | - 0.19 | 0.09 | 0.003 | 0.49 |
| Age at onset | - 0.17 | 0.11 | 0.001 | 0.49 | |
| Disease duration | - 0.14 | 0.16 | 0.009 | 0.47 | |
| Control | Age | 0.1 | 0.23 | - 0.05 | 0.35 |
Table 3. Partial correlation between expressions of genes and clinical features (controlled for gender).
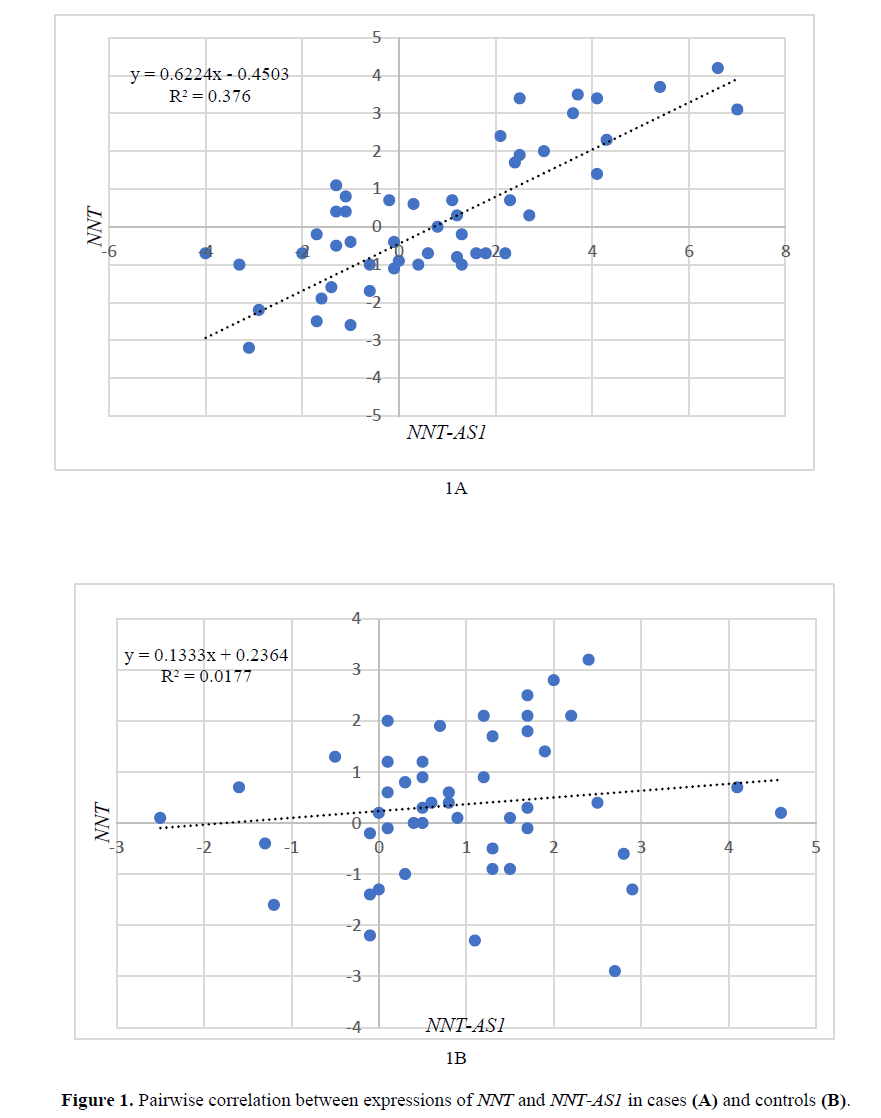
Pairwise correlation between expressions of NNT and NNT-AS1
Expression levels of NNT were significantly correlated with expression of NNT-AS1 in patients (R2=0.367, P=0.03) (Figure 1A). However, the expression of these genes were not correlated in healthy subjects (R2=0.01, p>0.05) (Figure 1B).
Discussion
Bipolar disorder is a psychiatric disorder with poorly clarified background. Several elements such as dysregulation of signaling pathways and gene expression, defects in synaptic plasticity, diminished cellular flexibility, decreased brain cell mass, and aberrations in brain structure and function are suggested to participate in its pathogenesis (Chiu et al., 2013). Previous animal and human studies have also demonstrated the role of oxidative stress in the pathogenesis of bipolar disorder (Ng et al., 2008).
For instance, dysregulation of superoxide dismutase (SOD) activities have been detected in bipolar patients (Abdalla et al., 1986, Kuloglu et al., 2002). Moreover, the levels of the lipid peroxidation products thiobarbituric acid reactive substances (TBARS) have been higher in bipolar patients compared with healthy subjects (Kuloglu et al., 2002). Another study has shown high TBARS levels in bipolar patients irrespective of disease phase, whereas GSH-Px activity was only raised in euthymia but not in depressed or manic phases. Conversely, elevated SOD activity was detected in both manic and depressive phases but not in euthymia (Andreazza et al., 2007). Despite extensive efforts in linking the oxidative stress and bipolar disorder, the role of NNT as a major determinant of redox balance has not been evaluated in bipolar disorder. In the present study, we evaluated expression of NNT and NNT-AS1 in peripheral blood of bipolar patients and healthy subjects. NNT has role in function of mitochondria. Nnt-deficient cells had more levels of oxidized mitochondrial peroxiredoxin, which made them more sensitive to persistent rises in H2O2 and cell death after contact with paraquat (Lopert and Patel, 2014). Meanwhile, several lines of evidences point to dysfunction of mitochondria as an underlying cause of bipolar disease (Kato and Kato, 2000). However, we found no significant difference in expression of NNT and NNT-AS1 between these two groups which might be due to the fact that all patients were in euthymic phase. Based on the results of previous studies, expression of genes might be different in manic, depressive and euthymic phases. There are evidences for intrapersonal changes in gene expression between depressed and euthymic phases (Munkholm et al., 2012). Moreover, expressions of few genes have been shown to be different between manic and depressed phases (Munkholm et al., 2012). Consequently, assessment of NNT and NNT-AS1 in different phases of bipolar disorder would help to explore their role in the pathogenesis of bipolar disorder. Moreover, the role of administered mood stabilizers in regulation of these genes cannot be ruled out. A previous study has assessed differentially expressed genes in postmortem brains from bipolar patients and controls using microarray technique and found no difference in gene expressions between controls and patients who received antipsychotic treatments which implied that these drugs would bring the level of altered genes to the normal state (Chen et al., 2013). Another study in bipolar patients have demonstrated the role of lithium in changing expression of numerous immune- and signal transduction-related genes leading to the proposition of peripheral gene expression as a biomarker of lithium function (Anand et al., 2016).
We also detected no significant correlation between relative expression of genes and age of study participants which demonstrates the steady-state of their expression during aging at least in the age range of study participants. Future studies are needed to assess correlation between their expression and age in other age ranges. Moreover, expression of either gene was correlated with age at disease onset or disease duration in cases. The significance of this finding can only be discussed if future studies reveal dys-regulation of their expression in manic or depressed phases of bipolar disease.
Finally, we found significant correlation between expression levels of NNT and NNT-AS1 in patients despite lack of correlation between their expressions in healthy subjects which might imply the role of NNT-AS1 in enhancing stability of NNT especially in the context of bipolar disorder. Several naturally occurring anti-sense RNAs have been shown to alter the expression of their sense transcripts. The expression levels of sense/antisense pairs are mostly concordant, but discordant pattern is also reported (Fang and Fullwood, 2016). The pattern of correlation between NNT and NNT-AS1 in our study is concordant and similar to most of other reported studies. However, functional studies are needed to elaborate the mechanism of action of NNT-AS1 on expression of NNT.
Conclusion
In brief, lack of difference in the expression of NNT and NNT-AS1 between bipolar patients and healthy subjects as demonstrated in our study does not rule out the influence of these genes in the pathogenesis of this disorder. Future studies are needed to assess intraindividual differences in their expression in distinct disease phases.
Acknowledgment
This study was financially supported by Shahid Beheshti University of Medical Sciences.
Conflicts of interest
The authors declare they have no conflict of interest.
About the Authors
Corresponding Author
Mohammad Taheri
Student Research Committee, Shahid Beheshti university of Medical Sciences, Tehran, Iran
- Email:
- Mohammad_823@yahoo.com
Arezou Sayad
Department of Medical Genetics, Shahid Beheshti university of Medical Sciences, Tehran, Iran
- Email:
- infolib@sbmu.ac.ir
References
- Abdalla DS, Monteiro HP, Oliveira JA, Bechara E J (1986) Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem 32: 805-807.
- Anand A, Mcclintick JN, Murrell J, Karne H, et al., (2016) Effects of Lithium Monotherapy for Bipolar Disorder on Gene Expression in Peripheral Lymphocytes. Mol Neuropsychiatry 2: 115-123. https://doi.org/10.1159/000446348
- Andreazza AC, Cassini C, Rosa AR, Leite MC, et al., (2007) Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res 41: 523-529. https://doi.org/10.1016/j.jpsychires.2006.07.013
- Association AP (2013) Diagnostic and statistical manual of mental disorders (DSM-5®), American Psychiatric Pub. https://doi.org/10.1007/springerreference_179660
- Chen HM, Wang NL, Zhao X, Ross CA, et al., (2013) Gene expression alterations in bipolar disorder postmortem brains. Bipolar Disorders 15: 177-187. https://doi.org/10.1111/bdi.12039
- Chiu CT, Wang Z, Hunsberger JG, Chuang DM (2013) Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65: 105-142. https://doi.org/10.1124/pr.111.005512
- Fang Y and Fullwood MJ (2016) Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics 14: 42-54. https://doi.org/10.1016/j.gpb.2015.09.006
- Ho HY, Lin YT, Lin GG, Wu PR, et al. (2017) Nicotinamide nucleotide transhydrogenase (NNT) deficiency dysregulates mitochondrial retrograde signaling and impedes proliferation. Redox Biology 12: 916-928. https://doi.org/10.1016/j.redox.2017.04.035
- Hoek JB and Rydstrom J (1988) Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J 254: 1-10. https://doi.org/10.1042/bj2540001
- Jackson JB, Leung JH, Stout CD, Schurig-Briccio, LA et al., (2015) Review and Hypothesis. New insights into the reaction mechanism of transhydrogenase: Swivelling the dIII component may gate the proton channel. FEBS Lett 589: 2027-2033. https://doi.org/10.1016/j.febslet.2015.06.027
- Kato T and Kato N (2000) Mitochondrial dysfunction in bipolar disorder. Bipolar Disord 2: 180-190. https://doi.org/10.1034/j.1399-5618.2000.020305.x
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, et al., (2002) Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct 20: 171-175. https://doi.org/10.1002/cbf.940
- Lopert P and Patel M (2014) Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem 289: 15611-15620. https://doi.org/10.1074/jbc.m113.533653
- Munkholm K, Vinberg M, Berk M, Kessing LV (2012) State-related alterations of gene expression in bipolar disorder: a systematic review. Bipolar Disord 14: 684-696. https://doi.org/10.1111/bdi.12005
- Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11: 851-876. https://doi.org/10.1017/s1461145707008401
- Wang Q, Yang L, Hu X, Jiang Y, et al., (2017) Upregulated NNT-AS1, a long noncoding RNA, contributes to proliferation and migration of colorectal cancer cells in vitro and in vivo. Oncotarget 8: 3441-3453. https://doi.org/10.18632/oncotarget.13840
Keywords:
Download:
Full PDF- Share This