Electroporation induced membrane permeability changes at the ionic level
Received: July 30, 2019
Accepted: August 17, 2019
Published: September 05, 2019
Genet.Mol.Res. 18(3):
Micro Abstract
In this study, we evaluate and measure ionic currents across the cell membrane before and after the process of electroporation, and understand the recovery time of the membrane on human breast cancer cells after delivery of electroporation pulses.
Abstract
Background/Aim: Electrochemotherapy is an innovative method for the localized treatment of cutaneous and subcutaneous metastatic cancers. The aim of the study is to assess the changes at the plasma membrane and its recovery time by monitoring the regulated passage of ions through specific ion channels as measured by electrophysiology.
Materials and Methods: Electroporation and patch clamp techniques were used to study the effects of electric pulses on the cell membrane.
Results and Discussion: While there is an exclusion of large macromolecules like Trypan Blue in just 10 minutes after electroporation, the cell membrane however still permits non-specific passage of ions across the membrane upto and even after 30 minutes after electroporation.
Conclusion: Cell death in reversible electroporation could be due to signaling events which act within and upto 24 hours after the pulse is delivered and not due to cell death instantaneously.
Keywords
Cancer cell; Electroporation; Patch clamp technique; Trans membrane potential; Membrane; Ionic current; Transient pores; Membrane resealing
Introduction
Cancer is a condition characterized by uncontrolled cell division which results in almost 13% of all human deaths. As projected and presented by GLOBOCAN (an International Agency for Research on Cancer), there is an ever-increasing incidence of cancer, as observed in 2008, when there were 4,690,000 cases of breast cancer and 4, 50, 000 cases of cervical cancer (https://www.cancer.org/, http://gco.iarc.fr/). It is also predicted that there would be surge in the number of cases of cancer with a projection of at least 1.1 million cases of breast cancer and 7, 30, 000 cases of cervical cancer. The cancer deaths in less developed countries of the world are expected to overtake those in the developed regions of the world as the death percentage with an increase from the current 88% to 99% in cervical cancer patients and from 59% to 63% in breast cancer patients. The prevalent misconception is that breast cancer is principally a crisis of developed countries rather than of developing countries, though the fact remains that the majority of the world’s 425 000 deaths from breast cancer occurred in developing countries (as defined by Forouzanfar et al.). Of the total breast cancer mortalities that occurred in developing countries, 68000 occurred in women less than 50 years. The additional financial burden on cancer patients makes the management of treatment strategies currently adopted seem difficult.
A majority of the side effects associated with chemotherapy could be attributed to overdose of the drugs primarily due to the non-permeable nature of the cell membrane. Much of this could be alleviated by making the cell membrane more permeable to permit the entry of the chemo drugs into the cytosol. One technique developed recently revolves around the principle of electroporation, or in other words, usage of short duration electric pulses to temporarily open up pores in the cell membrane through which the drugs can enter the cell. This technique called electro chemotherapy has been very successful in treating a wide variety of surface tumours (Weaver JC, 2000).
To elaborate on this technique, one needs to be aware of the cell, its permeability, and the changes that occur when electric pulses are applied. The cell membrane is a bilayer of lipids interspersed with proteins which protect the cell from its environment. Most of these proteins are ion channels/ pumps or transporters which aids in the transport of molecules across the plasma membrane. The lipids are held by weak non-covalent interactions inspite of which it remains a very stable structure.
Electrically speaking, the plasma membrane can be viewed as a thin insulator enveloped on either side by electrolyte solutions (the cytosol and the extracellular milieu). When exposed to electric fields of sufficient intensity, the membrane breaks down and there is a large increase in the electrical conductivity and thereby the permeability of the same. This process is called membrane electroporation (Sundararajan R et al., 2011). Unlike conventional insulators, where electric breakdown causes a permanent damage, the cell membrane has the ability to spontaneously return to its pre-breakdown state due to the behaviour of the lipids as a 2-D liquid and its resultant fluidity.
While there have been several reports on the formation of unstable extremely transient aqueous pores in the lipid bilayer, electric pulses by means of induction of a voltage difference across the bilayer, reduces the energy needed for the generation of metastable pores which remains milliseconds to minutes after the electric field is removed (Haltiwanger S, 2003).
When the electric field to which the cell is exposed to is short, the membrane retains the ability to recover rapidly and the cell remains viable; this process is termed as reversible electroporation (Coster HGA, 1965). However, upon application of high-intensity pulses of longer duration, the cell membrane irreversibly breaks down and is called as irreversible electroporation. Both types of electroporation have been used immensely in both the clinical set up as well as in biotechnology research. Irreversible electroporation using high-intensity electric pulses for longer duration is used in electrical ablation of tissues as in biopsies, sterilization etc. Reversible electroporation has a wide variety of applications including electro chemotherapy, gene therapy etc. in medicine and to extract biomolecules, sterilization (Sauvé R et al., 1983).While there is much known about the clinical and research significance of this technique, details at the membrane and cellular level remain yet to be understood. For instance, while there are reports indicating that pores are formed in several nanoseconds according to Molecular Dynamics simulations, experimentally it has been demonstrated that there is a delay of several microseconds before membrane transport could be measured after the application of electrical pulses (American Joint Committee on Cancer, 2010). Additionally, membrane integrity is conventionally measured using Dye uptake experiments the most common and simple of which is the Trypan Blue uptake assay. In this, the viability of a cell is evaluated by measuring the ability of the cell to take in the dye to which it is normally impermeable to. If Trypan blue remains excluded as visualized microscopically, it implies that the cell remains viable as compared to the ones with membrane breakdown wherein the dye gets into the cytosol thereby staining the cell blue. While this is a good indicator of viability, it, however, does not display an accurate state of the membrane with respect to membrane strength and ionic transport across the same after the process of transient membrane breakdown. This information could be pivotal in identifying the exact processes which the membrane undergoes with respect to transport phenomenon as well as the integrity of the membrane. This can ideally be measured using electrophysiological studies which have been attempted here.
With the wide prevalence and increasing incidence of cancer worldwide, it is imperative that effective treatment methods are developed to inhibit its spread. Progressive research is now reconnoitring the possibilities of effective targeted drug delivery. Electro chemotherapy is one such effective method, where electric pulses are applied at the tumour site to increase drug delivery to the cancerous cells. For these studies, one of the initial drugs used was bleomycin, a hydrophilic drug with limited permeability through the membrane of the cell, but a highly cytotoxic drug once bound to DNA. Using this procedure targeted delivery of the cytotoxic drug is viable, with improved drug effectiveness at lesser dosages. With lesser dosages (made possible because of electro chemotherapy), side effects due to these cytotoxic drugs are either nil or minimum as observed in trials on human and animal patients.
In spite of numerous theoretical studies by electrical circuit modeling, numerical simulations and molecular dynamics on this phenomenon the mechanisms of electroporation and the subsequent electroporation-induced biological phenomena could not be understood fully. There exist two hypotheses on the mechanism underlying the membrane level changes during the process of electroporation:
I. The long duration hydrophilic pores allow the passage to the previously inhibited molecules through the cell membrane
II. The alternate theory supported by molecular dynamics calculations, explains that water molecules enter the defects of the membrane, resulting from the application of the external electric field, facilitating the passage of molecules (e.g. bleomycin) due to the enhanced hydration and permeability of the membrane.
In spite of limited knowledge about the mechanism through which electroporation increases the sensitivity and permeability of the membrane, its applications on electro chemotherapy, tumor ablation, sterilization and food processing are immense. Hence it is imperative to understand the mechanism thoroughly in a quest to develop the most ideal pulse protocol to achieve the same.
Patch clamp electrophysiology is a method used to study the process of ionic transport across the cell membrane. It is a highly sophisticated technique involving the isolation of a patch of the cell membrane by forming an electric seal with resistance in the order of giga ohms followed by clamping the membrane at a particular voltage and then measuring the ionic currents (in the voltage clamp method) or by studying the voltage changes across the membrane in response to a fixed current delivered to the isolated patch (in the current clamp method (Karmazinova M et al., 2010). This method requires a very high integrity of the cell membrane with minimal ‘leak’ currents which make the membrane ‘not clampable’. This could hence be used as an indicator of the health of the membrane which could be more accurate than the dye exclusion assay. Here we compare both the techniques and measure ionic currents across the cell membrane before and after the process of electroporation to also try and understand the recovery time of the membrane (Li M et al., 2011). This study is preceded with an analysis of the effect of extreme electrical pulses in irreversible electroporation and the expression changes in the levels of proliferation markers like PCNA. This was studied on Lung Adenocarcinoma cell lines (A549) by varying the different electrical parameters in order to characterize the optimal values for irreversible electroporation. The parameters analysed in the study include varying electric field, number of pulses and pulse length of each pulse and these variables influence the cell viability to various extents.
Materials and Methods
Cell culture
A549, HBL-100 and HeLa cell lines were purchased from National Centre for Cell Science (NCCS), Pune, India at. All experiments were carried out within 20 additional passages. Cells were grown in DMEM (Dulbecco's Modified Eagle Medium) with L-Glutamine and Sodium pyruvate supplemented with 10% with fetal bovine serum (FBS) and 1% with a mixture of penicillin (5000 U/ml)/streptomycin (5000 Ag/ml). Cells were grown under controlled conditions at 37°C and 5% CO2. PCa cell line, DU145 was purchased from the American Type Culture Collection (ATCC). It is androgen insensitive and highly metastatic cell line derived from brain metastases. The cells were cultured in Eagle's Minimum Essential Medium (GIBCO, Langley, USA) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO), at 37°C in a humidified atmosphere containing 5% CO2.
Electroporation
A cell suspension of (3.5 × 105 cells/ml) was taken in a 4mm cuvette for electroporation using the BTX ECM 830 square wave electroporator (BTX, San Diego, CA). The parameters are set on the instrument according to the instructions provided in the instrument manual. The electroporation buffer used was DMEM with 5% FBS. Since no drug is used, 300 μl of the cell suspension alone was used for electroporation. The values selected for electric field are 500 V/cm, 1500 V/cm and 2500 V/cm. The pulse length range and the number of pulses used in the experiments are 200 μs, 250 μs, 300 μs and 20, 40, 60 respectively. The standardization of electric field and number of pulses were carried out using CEF whereas the variations of pulse length were normalised using A549 cell line. In the number of pulse experiments, a single train has 10 pulses with frequency of 1Hz and accordingly 2, 4, 6 and 8 trains were used for 20, 40, 60 and 80 pulses respectively. An interval of 60 seconds was given between each train (Andreason GL, 1993).
Dye exclusion assay
Cells were harvested from the 35-mm dishes by treatment with 0.5% trypsin-EDTA and incubating them at 37°C for 5 minutes. The cells were centrifuged at 1000 rpm for 1 minute and then resuspended in 1 mL of culture medium. A ratio of 1:1 of trypan blue and the cell suspension was mixed of which 10 μl was seeded into a precleaned Neubauer chamber (Nexcelom Bioscience LLC. MA). Cells were observed at 40x magnification of a light microscope. Cell viability is computed as the ratio of a number of live cells to the total number of cells present (both Dead and Live cells) (Strober W, 2001).
Electrophysiology
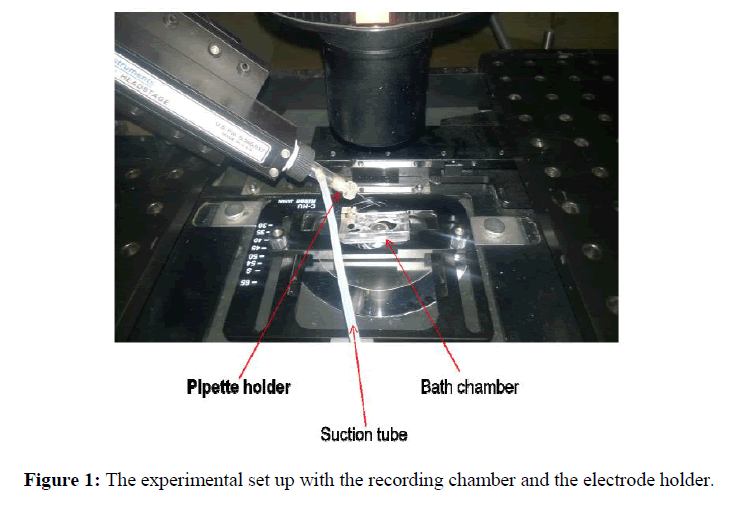
Pipettes were pulled on a Sutter P-87 Glass Puller from thick-wall borosilicate glass (Outer Diameter (OD) 1.5 mm, Inner Diameter (ID) 1.1 mm, and 10 cm length)with the following program; Heat 500, Pull 0, Velocity 40, Time 250. The puller was equipped with a box-type filament. A typical pipette had an overall length of 5 cm, a cone length of ~4 mm, and an orifice diameter of ~1-2 μm. For single current measurements, the coverslips were placed into a working chamber filled with a solution containing (mmol/1): 140 KCL, 2 CaCl2, 2 MgCl2, 20 HEPES, 10 glucose. pH is adjusted to 7.3 with KOH and the osmolality is adjusted to 300 mOsms with glucose. Single-channel currents were recorded using cell-attached configuration. Current signals were stored on analog tape and digitized for analysis. Glass pipettes were filled with a solution containing (mmol/1): 119 KCL, 2 CaCl2, 2.9 MgCl2, 10 HEPES (Sigma). After patch excising the pipette was moved to the small volume compartment (a glass tube of the volume 0.01 ml) of the chamber (Figure 1). Our experiments were performed with Axopatch 200B amplifier where appropriate filters are provided within the amplifier. The current was signals observed with the patch clamp amplifier and filtered with analog filtering device to improve the signal to noise ratio. Analog signal was converted to digital form by A/D converter (Digidata). Digital signal from Digidata was sent to a computer to provide waveform in the pClamp 10.3 software (Kotnik T et al., 2012; Sherman Gold R, 2002).
RNA isolation and Real time quantitative PCR
After electroporation, the cells were cultured with DMEM containing 5% FBS. After 48hrs of incubation, the total RNA was extracted from electroporated and control CEF using Medox- Easy spin column total RNA minipreps. The RNA was reverse transcribed in a 20 μl reaction mixture containing 2 μl of 10x buffer, 0.8 μl of dNTP, 2 μl of random primer, 1 μl of multitranscriptase, 4.2 μl of water and 10 μl of RNA sample for 10 min at 25°C, 2 min at 85°C and at 37°C for 2 hr. One μl of cDNA was then amplified in a mixture containing 5 μl of SYBR Green, 0.5 μl of forward primer, 0.5 μl reverse primer and 3 μl of water. The primers for PCNA and GAPDH gene were obtained from Sigma. The RT-PCR reaction program was 50°C for 2 min, 95°C for 10 min, 95°C for 15 sec and 60°C for 1 min. Forty cycles of amplification was performed in Applied Bio systems 7900HT. The results were then analyzed in SDS version 2.3 software (Heid CA et al., 1996).
Results and Discussion
Three different sets of studies were conducted in order to identify the optimal electric field, pulse length and number of pulses capable enough of promoting complete cell ablation. Percentage cell viability, a fraction of average number of live cells to total number of cells present was measured to check the efficiency of each parameter. The parameters that are considered are 2500 V/cm, 1500 V/cm and 500 V/cm for electric field; the pulse lengths taken into consideration are 300 μs, 250 μs and 200 μs; and numbers of pulses are 20, 40 and 60. Viability of cells for each of these parameters is plotted as a bar graph.
Effect of IRE on Lung Adenocarcinoma Cell Line (A549)
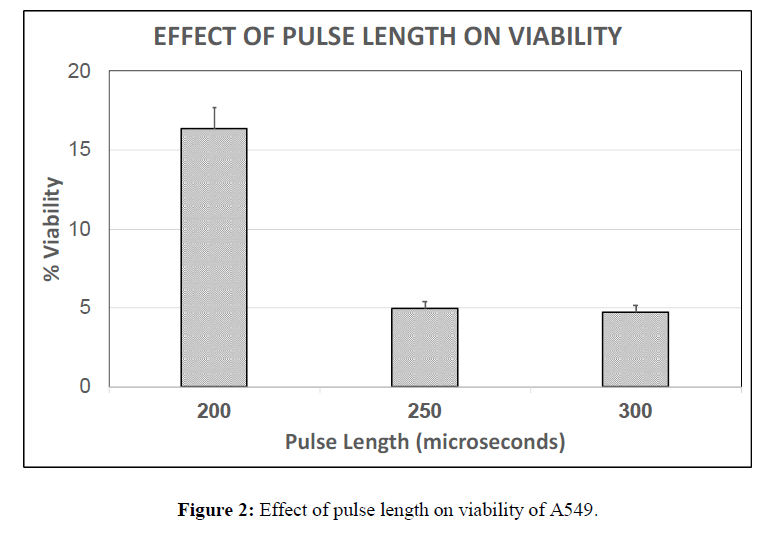
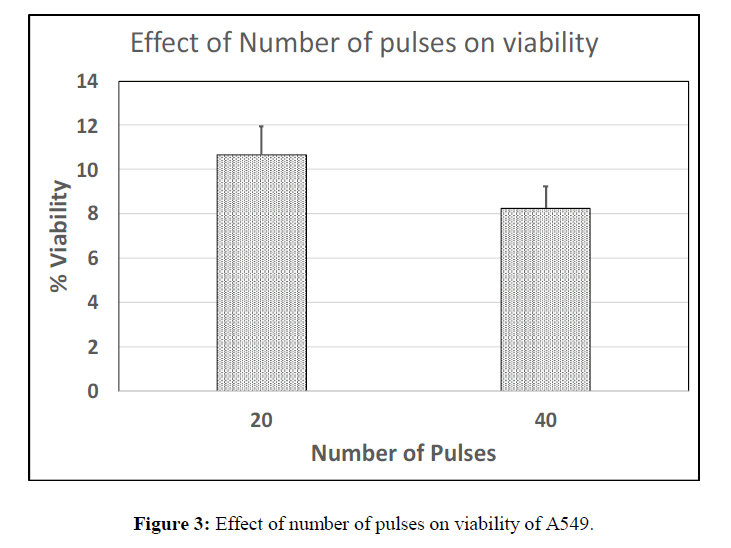
It is observed that the viability at 250 μs was slightly higher than 300 μs at constant electric field of 1500 V/cm hence this was chosen as the optimal parameter. It is also observed that the viability percentage at 200 μs is much higher than 300 μs and 250 μs of the cancer cell line (Figure 2) and also higher than that observed at 200 μs for chick embryo fibroblasts (data not shown), which shows that cancer cells are less sensitive to the pulse length as low as 200 μs. The viability as a function of the number of pulses was also studied. The viability of A549 cells for 60 pulses was completely zero where only cell debris was observed. The viability at 40 pulses was found to be lesser than that of 20 pulses with a difference of about 2%. Hence 40 pulses were chosen as a nominal number of pulses applied. (Figure 3).
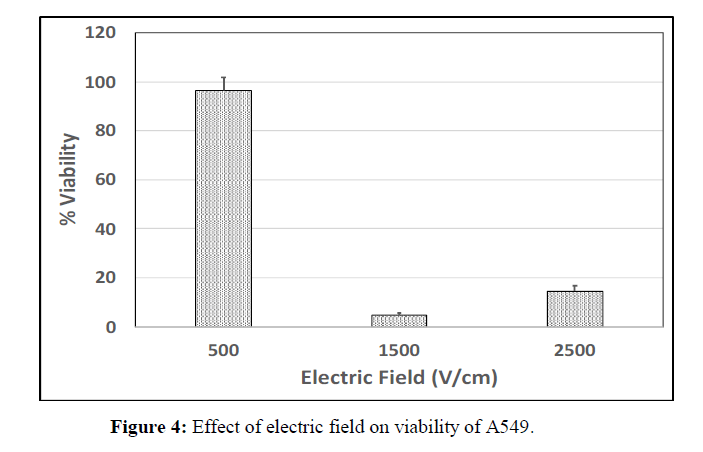
Electric field is one of the most important parameters which have a major impact on cell viability. The cancer cells were unresponsive to single pulse and hence 8 pulses were used in order to observe the viability response. The combination of 500 V/cm with 8 pulses is found to have very little effect on the cells with only 4% of cell death. Whereas, at 1500 V/cm, the viability percentage was observed to be very less when compared to all the other parameters (Figure 4).
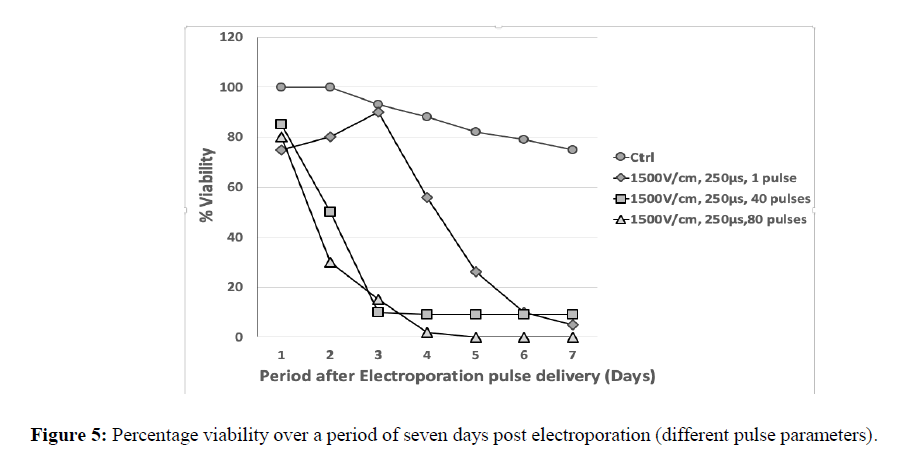
The growth curve of cancer cells, A549 (Lung Adenocarcinoma cells) was studied for three optimized parameters for electric field (1500 V/cm, 250 μs, 1 pulse), number of pulses (1500 V/cm, 250 μs, 40 pulses) and pulse length (1500 V/cm, 250 μs, 80 pulses). Initially there was a small increase in the growth of cells treated at 1500 V/cm, 250 μs, 1 pulse, but found to drop rapidly following the third day. But when the number of pulses was 80 with a field of 1500 V/cm there was decline in growth and found to cease completely by day 5 (Figure 5).
Quantification of PCNA gene expression
The effect of IRE on the cells at the molecular level was studied by quantifying the gene expression of PCNA (Proliferating Cell Nuclear Antigen). The cells entered the death phase only after day 4 following electroporation. Though the cells were not completely killed immediately following electroporation, the effect of IRE was observed after some days which is clear from the Viability percentage plotted against the duration after the IRE pulse and CFU assays (data not shown). The results of the studies show that IRE may have influence at the DNA and RNA level. To study whether IRE has an effect on gene expression or causes DNA damage, the expression level of PCNA was quantified in the cells electroporated with 500 V/cm, 8 pulses and 200 μs. The expression of PCNA was found to increase by 1.2 folds following electroporation (Table 1). This shows that the IRE has induced the DNA damage which in response has increased the PCNA expression level to promote DNA repair.
| PCNA .ct | GAPDH.ct | Δct | ΔΔct | 2-ΔΔct | |
|---|---|---|---|---|---|
| Control | 26.2771 | 23.4901 | 2.787 | 0 | |
| Irreversibly electroporated cells | 25.6188 | 23.1627 | 2.456 | -0.3309 | 1. 258 |
Table 1: Effect of IRE on PCNA expression.
Whole-cell recording on HeLa cells before electroporation
Patch clamp experiments on HeLa cells were performed using the whole-cell configuration. The coverslip with HeLa cells was placed in a bath chamber on an inverted microscope (Nikon Eclipse TE 2000- U) to perform electrophysiology. The bath chamber was perfused with a bath solution. The pipette was filled with the electrolyte solution and inserted in the pipette holder containing Ag\AgCl2electrode. A small tube was glued with pipette holder, which allowed application of suction and pressure to facilitate the formation of the gigaseal. To carry out the electrical measurement, the ensemble was connected to an amplifier (Axopatch 200B amplifier) via Ag\AgCl2 in electrolyte solution (Sersa G et al., 2011; Kumar R et al., 2009). Once the cell membrane is attached to the tip of the pipette, gentle suction was applied to form the ‘gigaseal’. The process of seal formation could be observed with pClamp 10.3 data acquisition software by monitoring the increasing resistance of the pipette as it approaches the cell, attaches to it and then forms the seal.
The seal resistance between membrane patch and pipette tip was >1GΩ. After the gigaseal is formed, an external command of 5mV was applied to rupture the cell membrane. Whole-cell measurements were performed atroom temperature (22-24°C) with a holding potential of -60 mV using the Axopatch 200B amplifier. The low pass filter on the amplifier for the current signal was set to give a cutoff of 1 kHz. Digitization was performed using Digidata and the current signals were recorded with pClamp 10.3 software. To evaluate the voltage dependence of current activation a series of depolarizing steps with increased amplitude was applied from the suitable holding potential (HP). For HeLa cells, the holding potential was maintained at -60 mV. Ionic currents in response to the depolarizing potentials applied were recorded from -80 mv to + 60 mv in 20 mv steps.
Recording ionic currents from HeLa cells after Electroporation
A low-intensity pulse of 200 V/cm was applied to achieve pore formation without cell death. The electric field intensity E and the duration of the pulse T, constitute a relationship, ET=k, a constant. This means if E is smaller, T has to be longer and vice versa. This condition was chosen based on previously reported in-vitro and in-vivo studies. Eight pulses at one-second interval were applied using BTX ECM 830, square wave electroporator with 4 mm electrode gap cuvette (Table 2) (Kumar R et al., 2009).
| Sample | Electrical field intensity ( V/cm) | Pulse Duration (ms) | Pulse Numbers | Pulse Intervals(s) |
|---|---|---|---|---|
| Control (no treatment) | 0 | 0 | 0 | 0 |
| EP-10 ms | 200 | 10 | 8 | 1 |
Table 2: Parameters used for electroporation.
The preparations for performing whole-cell recordings were done as described earlier. Upon gigasealformation, the pipette potential was changed to a negative voltage, and repetitive positive voltage pulses in steps of few millivolts were given. Pulses of suction were applied to the pipette interior until a sudden increase in the size of the capacitive transient was observed. Whole-cell experiments on HeLa cells after electroporation (EP) were performed 5, 10, 15 and 30 minutes after the procedure.
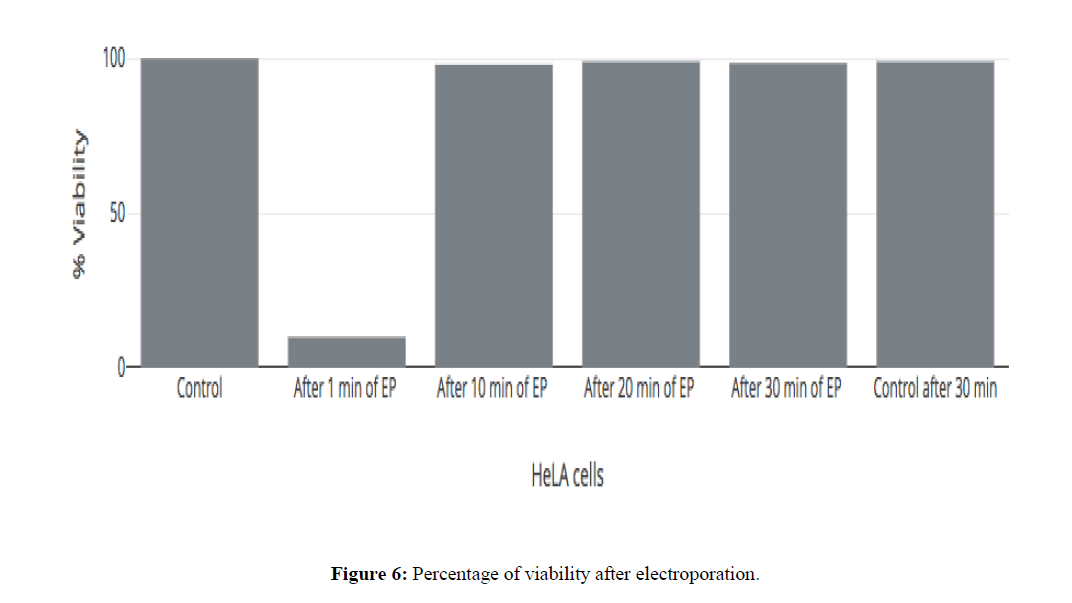
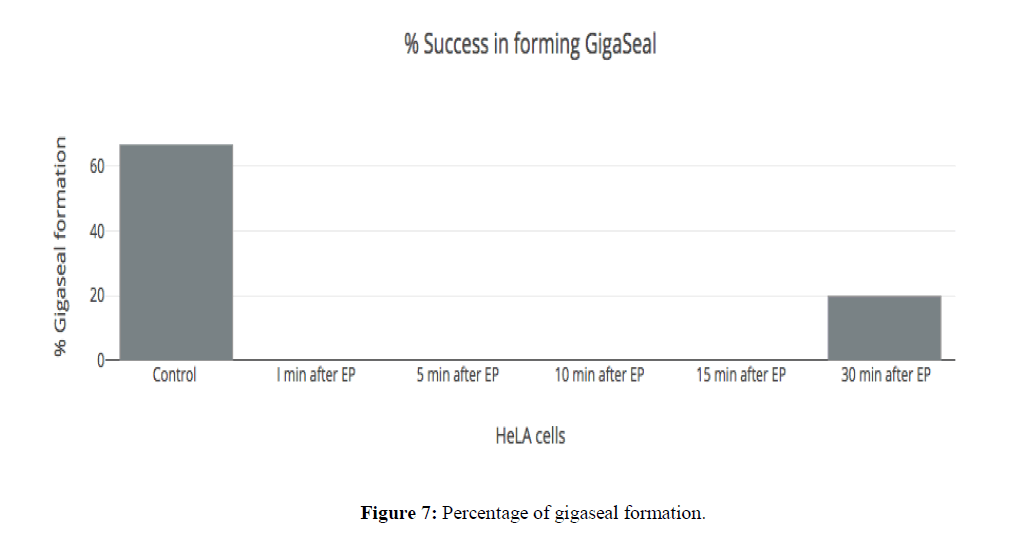
Glass pipette is moved towards cell with the help of micromanipulator then suction was applied, enabling membrane attachment to the tip of the pipette. Gentle suction was applied to form "gigaseal". The seal resistance between membrane patch & pipette tip was made <500 MΩ. None of the cells were able to be compatible with the gigaseal formation upto 15 minutes after electroporation due to the extreme high leak current (Figure 6 and Figure 7).
Only 20% of HeLa cells could form gigaseal after 30 minutes of electroporation (Figure 7). The rapid decline in the success rate of giga seal formation could be attributed to the formation of the pores in the membrane which permit a huge nonspecific passage of ions across the membrane. This is turn prevents the formation of a gigaseal due to the large amplitude of current passing across the membrane at all points thereby making it impossible to achieve an electrical sealing or insulation of the patch of the membrane which is the first step towards the formation of the giga seal and subsequent measurement of the ionic currents (Hille B, 2001).
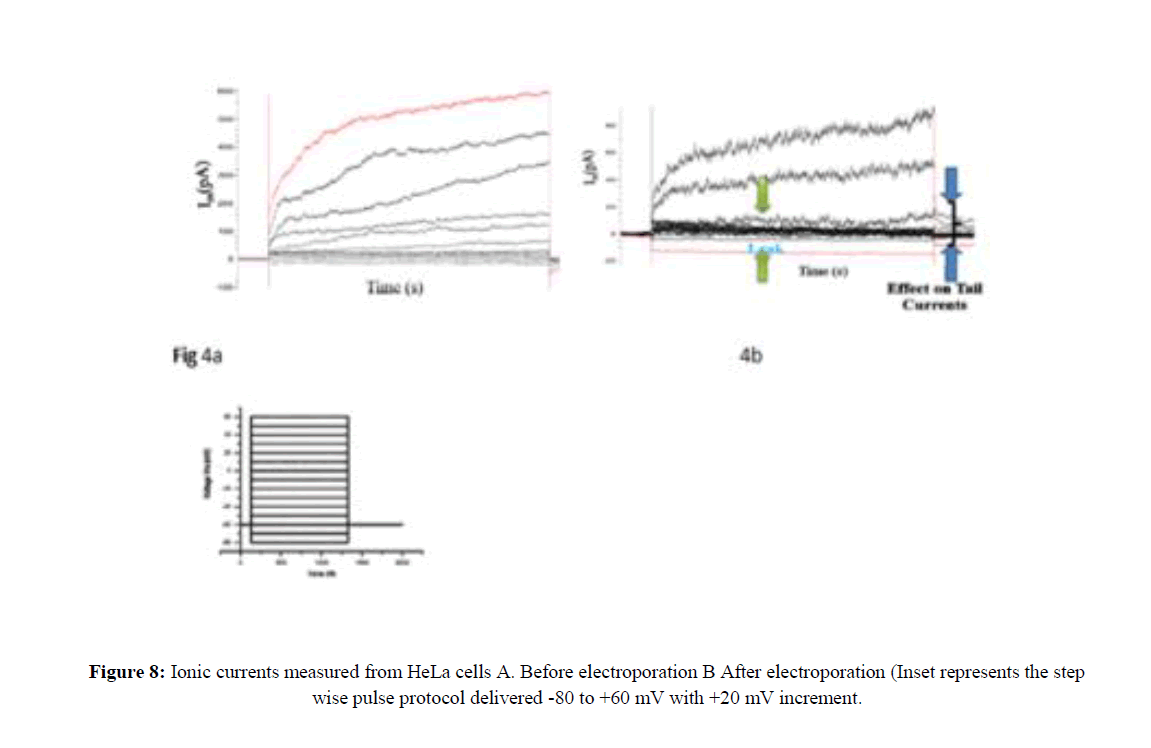
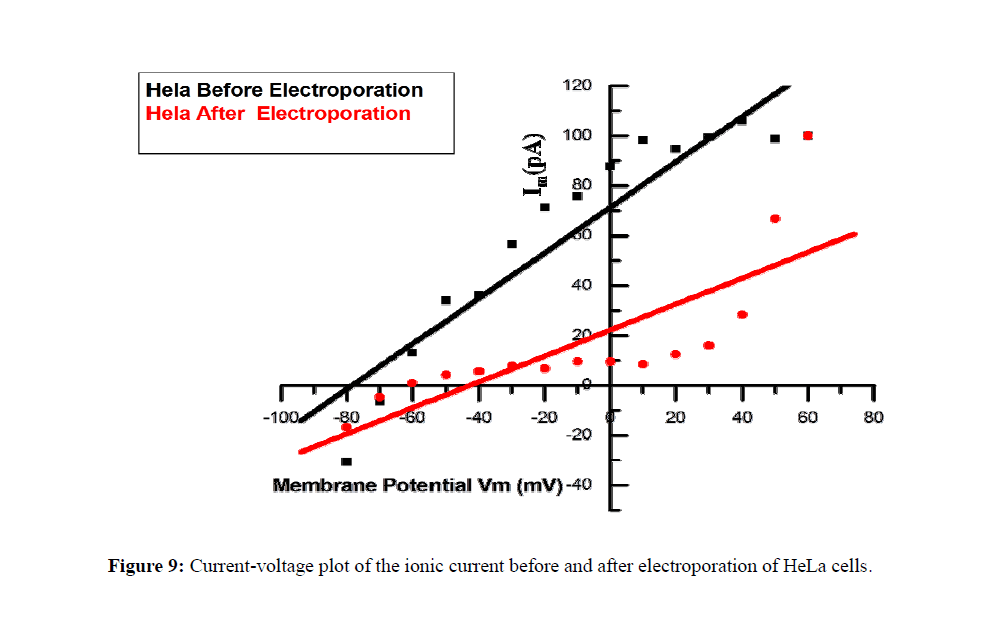
For same step voltage protocol, the current trace on the cell membrane after electroporation (EP) with holding potential of -60 mV (Figure 8). By electroporation, only 20% of the cell could form GΩ seal successfully. The very high nonspecific current was passing through membrane accounting for leak seen after electroporation. This leak was seen after 30 minutes of electroporation. The membrane does not seem to snap back to its original integrity after electroporation even after 30 minutes, post pulsing.
This implicates that at even 30 minutes post-electroporation, the membrane has not resealed completely to its pre-breakdown state as is evidenced by the high non-specific leak of ions across the membrane. At 30 minutes, there was close to 20% success in electrically isolating a patch of the cell membrane indicating that the non-specific leakage of ions ha started to diminish around that point by the commencement of closure of the non-specific metastable pores formed during EP (Mozhayeva GN et al., 1991; Mahaut Smith MP et al., 1989; Mccann JD et al., 1989; Sontheimer H et al., 2007; Suchyna TM, 2009).
Signaling pathway involving ions and electrical currents would be acting for a long duration, after pulse delivery which may account for programmed cell death. Figure 8 and Figure 9 shows the whole-cell ionic currents and their I/V relationship before and after electroporation of HeLa cancer cells. The amplitude of the current, as well as the signal to noise ratio, remained low in the post-electroporation recordings as compared to the control HeLa cells (Figure 10).
Trypan Blue Assay
Trypan blue assay was performed for the HeLa cells before and after electroporation to check the viability of cells in the time interval of 1minute up to 30 minutes. The electroporation parameters for the assay are shown in Table 2. While at one-minute post EP there where high levels of mortality, the cells seem to exclude trypan blue almost completely even at 10 minutes post EP indicating that the membrane pores generated had resealed enough to exclude the dye from the cytoplasm. It is found that there is no viability difference before and after electroporation. However, it was observed that the electroporated cells did not survive 24 hours after the delivery of the EP indicating that some signaling pathway had been activated which inspite of the membrane reseal had triggered cell death. These results hint that the membrane of the cell after electroporation reseals in as less as ten minutes to shut down permeability to large molecules like trypan blue (m=891.8 Da) as is evidenced by the close to 100% viability as estimated by the Trypan Blue Assay.
Limitation of the study
Although the principle of electroporation can be applied to all cell types and at all cell stages, its efficiency depends on the electrical properties of the cells and the tissue matrix it is present in which provide resistance to the application of effective dose of current. In addition, electroporation can lead to collateral damage when compared to some other physical methods. Since, electroporation field is applied through the skin using surface plate electrodes, the major potential drop develops across the skin instead across the targeted subcutaneous tissues that usually leads to skin edema. To circumvent this, we have in this study attempted to understand the mechanism in detail such that an optimal pulse with minimal amplitude and time duration need only be applied to the tissue thereby reducing the side effects.
Conclusion
Electroporation opens up pores in the membrane which increases the membrane permeability to ions and other biomolecules. The membrane reseals back in the case of reversible EP thereby making it impermeable to certain molecules. In this study, we categorized the permeability of the membrane to a large molecule like Trypan Blue and compared it with specific as well as nonspecific ionic permeability. It has been demonstrated that the membrane recovers to the extent of excluding Trypan Blue 10 min post EP. However, the membrane remains permeable to ions in a non-specific manner as evidenced by the high leak current in cells 30 minutes post EP even while it is impermeable to Trypan Blue. This could either implicate that there may be pores of different sizes of which the larger ones reseal rapidly or that the pores shrink in size gradually thereby cutting off permeability to bigger molecules as the recovery time increases. In addition, these results also signify that even though complete membrane resealing occurs, a signaling pathway for cell death is triggered as evidenced by the ability of the cell to be amenable to electrophysiological measurement but undergo cell death in 24 hours post EP. This could mean that it is not merely a membrane breakdown which causes the cell death but that the process triggers a cascade of molecular events that culminate in cell death. (Walz W, 1995; Wu YC et al., 1991; Pucihar G et al., 2008).
This study for the first time evaluates the after effects of electroporation pulses on the membrane currents which may have implications in the side effects that this technique may bring about. This could help devise customized pulse protocols to be used especially those to be used in the proximity of the excitable tissues of the body where the effect of ion channels are more profound. This needs to be explored in detail to gain an accurate understanding of the protocols which could safely be adapted for electro chemotherapy without causing much damage which may include tingling sensations etc. after the procedure and could possibly be attributed to the effect of this on the ion channels. Further studies to evaluate the molecular events may be pivotal in gaining a perfect understanding of the cell membrane and how EP affects the same.
About the Authors
Corresponding Author
Rajaprabu Ramachandran
B.S. Abdur Rahman Crescent Institute of Science and Technology, Vandalur, Chennai, India
Kavitha Sankaranarayanan
AU-KBC Research Centre, MIT Campus of Anna University, Chromepet, Chennai, India
- Email:
- skavitham@yahoo.com
References
- https://www.cancer.org/
- http://gco.iarc.fr/
- Weaver JC (2000) Electroporation of cells and tissues. IEEE Transactions on Plasma Science 28: 24-33. https://doi.org/10.1109/27.842820
- Sundararajan R, Rajendran R, Shahid SS, Santosh DK (2011) Effect of irreversible electroporation on cancer cells. IEEE Conference. https://doi.org/10.1109/ceidp.2011.6232622
- Haltiwanger S (2003) Electrical properties of cancer cells. Rife International Conference in Seattle.
- Coster HGA (1965) Quantitative analysis of the voltage-current relationship of fixed charge membranes and the associated property of “punch-through. Biophys J 5: 669-686. https://doi.org/10.1016/s0006-3495(65)86745-5
- Sauvé R, Roy G, Payet D (1983) Single channel K+ currents from HeLa cells. J Membrane Biol 74: 41-49. https://doi.org/10.1007/bf01870593
- American Joint Committee on Cancer (2010) Breast. In: AJCC Cancer Staging Manual, 7th ed. New York: Springer, 2010: 347-369. https://doi.org/10.1007/978-0-387-88441-7_32
- Karmazinova M and Lacinova L (2010) Measurement of cellular excitability by whole cell patch clamp Technique. Physiol Res 59: S1-S7.
- Li M and Xiong ZG (2011) Ion channels as targets for cancer therapy. Int J Physiol Pathophysiol Pharmacol 3:156-166.
- Andreason GL (1993) Electroporation as a technique for the transfer of macromolecules into mammalian cell lines. Journal of tissue culture methods 15: 56-62. https://doi.org/10.1007/bf01667362
- Strober W (2001) Trypan blue exclusion test of cell viability. Current protocols in immunology 21. https://doi.org/10.1002/0471142735.ima03bs21
- Kotnik T, Kramar P, Pucihar G, Miklavcic D (2012) Cell membrane electroporation Part 1: The Phenomenon. IEEE Electrical Insulation Magazine 28: 14-23 https://doi.org/10.1109/mei.2012.6268438
- Sherman Gold R (2002) EdInstrumentation for measuring bioelectric signals from cells. The Axon Guide, 2nd ed. Molecular Devices, USA, pp. 56.
- Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6: 986-94. https://doi.org/10.1101/gr.6.10.986
- Sersa G, Cemazar M, Snoj M (2011). Electrochemotherapy of solid tumors – preclinical and clinical experience. 33rd Annual International Conference of the IEEE EMBS Boston, Massachusetts USA. https://doi.org/10.1109/iembs.2011.6090165
- Kumar R, Muralitharen S, Usman SM, Ramachandran RP (2009) Electrically-enhanced proliferation control in adult human mesenchymal stem cells. Annual Report Conference on Electrical Insulation and Dielectric Phenomena. https://doi.org/10.1109/ceidp.2009.5377703
- Kumar R, Muralitharen S, Usman SM, Ramachandran RP (2009) Efficient and economical electro-drug delivery IEEE Toronto Conference. 843-848. https://doi.org/10.1109/tic-sth.2009.5444382
- Hille B (2001) Ion channels of excitable membranes, Third Edition, Sinauer Associates, Inc. Sunderland, Massachusetts, USA, p. 814.
- Mozhayeva GN, Naumov AP, Kuryshev YA (1991) Variety of Ca2+-permeable channel in human carcinoma A-431 cells. J Membrane Biol 124: 113-126. https://doi.org/10.1007/bf01870456
- Mahaut Smith MP and Schlichter LC (1989) Ca2+ activated K+ channels in human B lymphocytes and rat thymocytes. Journal of Physiology 415: 69-83. https://doi.org/10.1113/jphysiol.1989.sp017712
- Mccann JD, Li M, Welsh MJ (1989) Identification and regulation of whole cell chloride currents in airway epithelium. Journal of General Physiology 94: 1015-1036. https://doi.org/10.1085/jgp.94.6.1015
- Sontheimer H and Olsen ML (2007) Whole-cell patch clamp recordings. Neuromethods 38: 35-68. https://doi.org/10.1007/978-1-59745-492-6_2
- Suchyna TM, Markin VS, Sachs F (2009) Biophysics and structure of the patch and the gigaseal. Biophys J 97: 738-747. https://doi.org/10.1016/j.bpj.2009.05.018
- Walz W (1995) Perforated patch-clamp technique. Patch-Clamp Appl Protoc Neuromethods 26: 155-171. https://doi.org/10.1385/0-89603-311-2:155
- Wu YC, Rieger R, Sun Y (1991) Implementation of a neuron emulator for Patch-Clamp settings. Nature 354: 515-518.
- Pucihar G, Kotnik T, Miklavcic D, Teissie J (2008). Kinetics of trans-membrane transport of small molecules into electro-permeabilized cells. Biophys J 95: 2837-2848. https://doi.org/10.1529/biophysj.108.135541
Keywords:
Download:
Full PDF- Share This