Clinical and molecular findings in an Albanian family with familial adenomatous polyposis
Received: September 02, 2017
Accepted: September 28, 2017
Published: October 05, 2017
Genet.Mol.Res. 16(4): gmr16039823
DOI: 10.4238/gmr16039823
Abstract
Purpose: Familial adenomatous polyposis is an inherited precancerous condition characterized by multiple colorectal polyps. This brief report describes three generations of a family with a history of colorectal cancer in which genetic testing was useful for detecting individuals at risk. Methods: A next generation sequencing custom gene panel comprising 11 genes associated with familial adenomatous polyposis and colorectal cancer was used to screen the proband. Sanger sequencing was used for family segregation study. Results: Genetic testing showed that the proband, her affected sister and asymptomatic son carried the p.(Gln1338*) variant in the APC gene. Conclusion: APC genetic variations are the most frequent cause of familial adenomatous polyposis and are inherited with autosomal dominant transmission. According to the literature, the variation found in this family is associated with the condition and lies in a known mutation cluster region. Pedigree analysis suggested that the proband’s son should be included in a regular surveillance program to monitor for development of the disease and avoid more serious consequences. To our knowledge, this is the first clinical and genetic report of the condition described in an Albanian family.
Introduction
Familial adenomatous polyposis (FAP) is a highly penetrant inherited disorder characterized by development of multiple colon polyps that manifest at a very young age, generally between 15 and 35 years (Dalavi et al., 2015). If untreated, the disease progresses to colorectal cancer, leading to death at a mean age of 42 years (Campbell et al., 1994). In the European Union, the prevalence of the disease has been estimated at 1/11300-37600 (Half et al., 2009).
The disorder can be inherited by autosomal dominant transmission, caused by germline heterozygote variations in the APC gene (Groden et al., 1991), or by recessive transmission, caused by germline homozygote or compound heterozygote variations in the MUTYH, MSH3 and NTHL1 genes, though other genes are suspected to be involved (Sampson et al., 2003; Weren et al., 2015; Adam et al., 2016).
Prophylactic surgery is always required to prevent cancer, and is preferably performed before the age of 25 years. Surgical options for FAP patients depend on the number of rectal polyps and family history, and can range from subtotal colectomy to total proctocolectomy (Dalavi et al., 2015).
Here we report the case of a family affected with FAP. The case demonstrates the importance of early intervention combined with genetic testing extended to all relatives at risk for the purpose of monitoring and prophylaxis. To our knowledge, this is the first surgically and genetically documented case from Albania reported in the literature.
FAMILY STUDY
In this family, two sisters have FAP and their mother died of colon cancer in 2006. The elder sister, age 38 years, sought medical attention elsewhere due to blood in the stools and iron deficiency anemia. In 2016, coloscopy showed many polyps, some large in size, in the intestinal tract between the anus and the caecum. The polyps were compatible with a diagnosis of FAP. In the anal region, a crescent shaped occlusive mass caused moderate stenosis. Samples were obtained for histological examination, which revealed pT3pN2b adenocarcinoma (0/164), Astler Coller stage C2 with metastases to nine inferior mesenteric lymph nodes, and various adenomatous polyps with high to low grade dysplasia, compatible with the genetic syndrome of FAP. The patient underwent abdominal-perineal resection the same year. A CT scan showed absence of distant metastases. Two months after surgery, the patient underwent two cycles of chemotherapy with capecitabine and oxaliplatin (XELOX) every 3 weeks followed by postoperative radiotherapy with capecitabine. In 2017, gastroscopy showed an absence of pathological lesions of the oesophagus, stomach and duodenum.
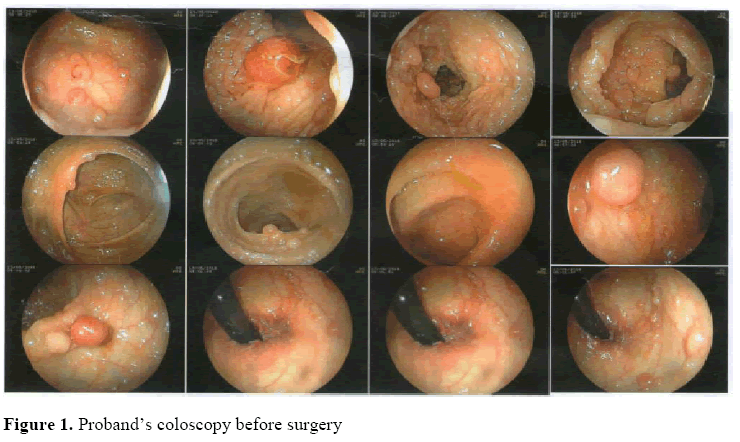
The proband, age 31 years, is the younger of the two sisters. In 2016, after her sister’s operation, she underwent coloscopy (Figure 1).
One year later she was operated for FAP with total colectomy and ileorectal anastomosis. The operation was performed by median xipho-pubic laparotomy. Exploration of the peritoneal cavity only revealed diffuse mesenteric lymphadenopathy of many pericolic lymph nodes, and to a lesser extend of the principal lymph nodes. On the transverse colon there was an area of about 2 cm adhering to the greater gastric curvature, indistinguishable from a neoplastic infiltration or inflammatory adhesion. A small portion of the gastric tract was resected en bloc and double sutured with 3-0 vicryl using a GIA 75 mm stapler.
Total colectomy was also performed including the main lymph nodes, followed by latero-terminal ileorectal anastomosis. The low portion of the rectum was involved by few polips; the patient was scheduled for endoscopic removal and endoscopic follow-up. Surgical material sent to the pathologist consisted of 60 cm of resected colon containing many polyps measuring 2-5 mm to 1 cm. The pathology report showed:
1-2) Resections: various edematous polyps with slight to moderate dysplasia at the colon distal resection margin. Absence of in situ or invasive malignancy at the resection margin. Proximal margin of ileum clear.
3) Adhesion: the colon-stomach adhesion included fibrotic adhesions and a reactive but not malignant lymph node.
4-6) Polyp fragments: various edematous polyps with slight and severe dysplasia were observed in the resected colon. No vegetative, ulcerative or infiltrated areas were found. The polyps ranged in size from 2 to 2.5 mm.
7-9) Twenty-one reactive lymph nodes out of 21 examined.
The conclusion was adenomatous polyposis of the colon with slight and severe dysplasia but without evidence of malignancy.
Post-operative recovery was excellent. The patient suffered slight post-operative anemia but recovered completely within a period of about 3 months.
GENETIC EVALUATION
Considering the high heritability and the early age of onset of FAP, genetic testing was performed in all family members at risk. DNA was obtained from whole blood of six individuals: the two affected sisters and their children. All subjects were invited to sign a specific informed consent for case reports.
In the proband, genetic variations were screened in all coding exons and flanking exon/intron boundaries of the following genes: APC (NM_000038; OMIM *611731), AXIN2 (NM_004655; OMIM * 604025), BMPR1A (NM_004329; OMIM * 601299), ENG (NM_000118; OMIM * 131195), GREM1 (NM_001191322; OMIM * 603054), MUTYH (NM_001128425; OMIM * 604933), NTHL1 (NM_002528; OMIM * 602656), POLD1 (NM_002691; OMIM * 174761), PTEN (NM_000314; + 601728), SMAD4 (NM_005359; OMIM * 600993), STK11 (NM_000455; OMIM * 602216).
The genomic regions containing the exons and the splicing sites of the above genes were sequenced using the Illumina commercial kit, TruSight One sequencing panel, on an Illumina MiSeq platform (150 bp paired-end reads).
Bioinformatic analysis of the data was performed using an in-house pipeline to align the sequence reads with a reference genome, as well as variant calling, annotation, and variant filtering to remove benign single nucleotide polymorphisms (variants with allele frequency ≤ 0.03).
Public databases such as 1000 Genomes Project Database (http://www.internationalgenome.org/), Exome Variant Server (EVS) database (http://evs.gs.washington.edu/EVS/), Exome Aggregation Consortium (ExAC) database (exac.broadinstitute.org/) and the public database of single nucleotide variants (dbSNP, www.ncbi.nlm.nih.gov/SNP/) were used to filter and prioritize the variants and to check for allele frequencies, while the Human Gene Mutation Professional Database (HGMD) (http://www.biobase-international.com/product/hgmd) was used to identify genetic variations previously reported as pathogenic.
Genetic variations were confirmed by PCR coupled with direct sequencing of target regions. Sanger sequencing, in search of any specific genetic variants found in the proband, was also programmed for all relatives. All laboratory methods are available on request.
Results
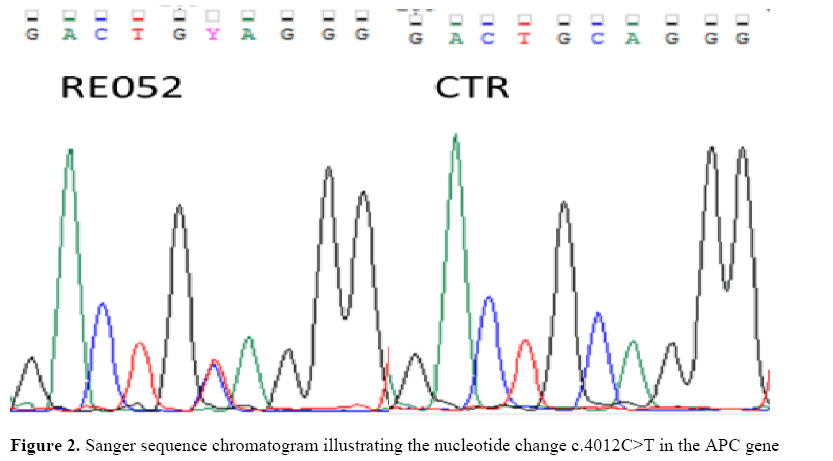
The mean coverage was 90.6X; 92.0% of regions had a coverage of at least 25X (coverage > 10X: 95.3%). The proband showed variant c.4012C>T or p.(Gln1338*) (rs121913327) in the APC gene (WNT signalling pathway regulator; previously known as adenomatous polyposis coli) (Figure 2).
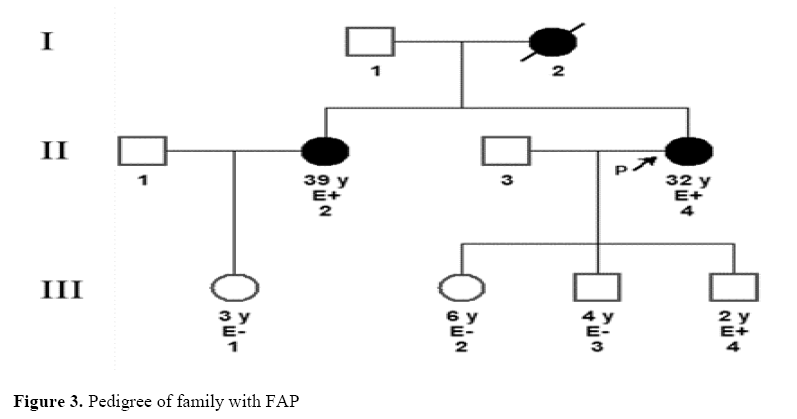
Sanger sequencing in search of the pathogenic APC variant in the family was positive in the case of the proband’s affected sister and one of the proband’s sons (Figure 3), in whom the disease has not yet manifested.
Legend: P, proband; E+ and E-, positive and negative to genetic test, respectively; square, male subject; circle, female subject; black symbol, affected subject; white symbol, healthy subject.
Discussion
FAP caused by genetic variants in the APC gene is an autosomal dominant disorder that predisposes to cancer. The APC protein normally acts as a negative regulator in the Wnt signalling pathway. Mutant APC protein does not break down β-catenin, leading to its accumulation. When translocated to the nucleus, accumulated β-catenin associates with the T-cell factor family of transcription factors, leading to uncontrolled cell growth and tumour development (Miyoshi et al.,1998; Bandipalliam, 2007).
The p.(Gln1338*) variant found in our proband leads to premature termination of the protein and is associated in the literature with both sporadic and familial cases of FAP (Groden et al., 1993; Friedl and Aretz, 2005). The variation lies near codon 1300 in exon 16, a well-known mutation cluster region (Miyoshi et al., 1992) associated with a particularly severe phenotype characterized by a large number of polyps and early onset of tumorigenesis (Nieuwenhuis and Vasen, 2007).
In the present family, two sisters had FAP; the elder underwent surgery after blood in the stools and anemia were detected. Unfortunately, the polyps degenerated into colorectal carcinoma. The proband presented without overt symptoms, persuaded by her family history: besides her sister, her mother also had FAP and died of colorectal cancer. She underwent surgery before malignancy developed. Genetic testing revealed that one of her sons is at high risk of FAP and he therefore began a regular surveillance program.
Almost complete penetrance (Bisgaard et al., 1994) and the fact that FAP may be asymptomatic before malignant transformation (Campbell et al., 1994) underline the need for appropriate monitoring and follow-up of all family members at risk. Genetic testing can unequivocally detect subjects at risk for the purpose of prophylaxis.
Usually, at risk individuals should undergo flexible proctosigmoidoscopy at age 10-12 years old and repeat it every 1-2 years until the age of 35. After the age of 35 the examination should be repeated every 3 years. An upper gastrointestinal endoscopy should be performed every 1-3 years starting from the moment when the first polyps are identified.
Conclusion
Moreover, it has been suggested that genetic testing may help make the decision between restorative proctocolectomy with ileal pouch-anal anastomosis and abdominal colectomy with ileorectal anastomosis. It has been observed that the risk for rectal cancer is higher in patients with an APC variations between codons 1250-1464 than in patients with variations outside this region (Nieuwenhuis and Vasen, 2007). This may influence the decision to offer abdominal colectomy with ileorectal anastomosis to those whose APC variation occurs proximal to codons 1250-1464 if proctoscopic examination reveals few polips in the rectum.
About the Authors
References
- Adam R, Spier I, Zhao B, Kloth M, Marquez J, et al. (2016). Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am. J. Hum. Genet. 99: 337-351. https://doi.org/10.1016/j.ajhg.2016.06.015
- Bandipalliam P (2007). Desmoid tumours and familial adenomatous polyposis: a tale of two syndromes. J. Clin. Gastroenterol. 41: 231-236. https://doi.org/10.1097/01.mcg.0000225659.38426.e7
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, et al. (1994). Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum. Mutat. 3: 121-125. https://doi.org/10.1002/humu.1380030206
- Campbell WJ, Spence RA, Parks TG (1994). Familial adenomatous polyposis. Br. J. Surg. 81: 1722-1733.
- Dalavi SB, Vedpalsingh TH, Bankar SS, Ahmed MH, et al. (2015). Familial adenomatous polyposis (FAP) - a case study and review of literature. J. Clin. Diagn. Res. 9: PD05-6. https://doi.org/10.7860/jcdr/2015/11636.5696
- Friedl W, Aretz S (2005). Familial adenomatous polyposis: experience from a study of 1164 unrelated German polyposis patients. Hered. Cancer Clin. Pract. 3: 95-114.https://doi.org/10.1186/1897-4287-3-3-95
- Groden J, Gelbert L, Thliveris A, Nelson L, et al. (1993). Mutational analysis of patients with adenomatous polyposis: identical inactivating mutations in unrelated individuals. Am. J. Hum. Genet. 52: 263-272. https://doi.org/10.1002/humu.9234
- Groden J, Thliveris A, Samowitz W, Carlson M, et al. (1991). Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 66: 589-600.
- Half E, Bercovich D, Rozen P (2009). Familial adenomatous polyposis. Orphanet J. Rare Dis. 4: 22. https://doi.org/10.1007/springerreference_35141
- Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, et al. (1998). Frequent mutations in the beta-catenin gene in desmoid tumours from patients without familial adenomatous polyposis. Oncol. Res. 10: 591-594.
- Miyoshi Y, Nagase H, Ando H, Horii A, et al. (1992). Somatic mutations of the APC gene in colorectal tumours: mutation cluster region in the APC gene. Hum. Mol. Genet. 1: 229-233. https://doi.org/10.1093/hmg/1.4.229
- Nieuwenhuis MH, Vasen HF (2007). Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit. Rev. Oncol. Hematol. 61: 153-161. https://doi.org/10.1016/j.critrevonc.2006.07.004
- Sampson JR, Dolwani S, Jones S, Eccles D, Ellis A, et al. (2003). Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 362: 39-41. https://doi.org/10.1016/s0140-6736(03)13805-6
- Weren RD, Ligtenberg MJ, Kets CM, De Voer RM, et al. (2015). A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 47: 668-671. https://doi.org/10.1038/ng.3287
Keywords:
Download:
Full PDF- Share This