Abstract
Effect of the beta secretase-1 inhibitor on the amyloid C-terminal fragment of amyloid precursor protein processing in a hyperphosphorylated tau rat model
Author(s): H.Y. Chen, L. Wang, J.F. Liu, W.Z. Wang and C.J. YuThe amyloid C-terminal fragment (βCTF) of the amyloid precursor protein (APP) is the cleaved component of APP by beta secretase-1 (BACE1), which shows similar neurotoxicity as amyloid beta (Aβ) in many ways. Evidence suggested that in addition to Aβ, βCTF might also participate in the pathogenesis of Alzheimer’s disease (AD). In recent years, the relationship between βCTF processing and hyperphosphorylated tau has attracted increasing research attention. In this study, we established an animal model of tau hyperphosphorylation with okadaic acid (OA) treatment, and analyzed βCTF processing in vivo. The βCTF level was found to increase in neurons, which was most likely caused by the induction of OA and BACE1 overexpression. Furthermore, these results provide the first evidence that βCTF can predominately accumulate in the axons of neurons in a hyperphosphorylated tau state in vivo, and suggested that the redistribution of βCTF is involved in the pathogenesis of AD. These results indicate that BACE1 could be a therapeutic target of AD by affecting the processing of βCTF.
Impact Factor an Index

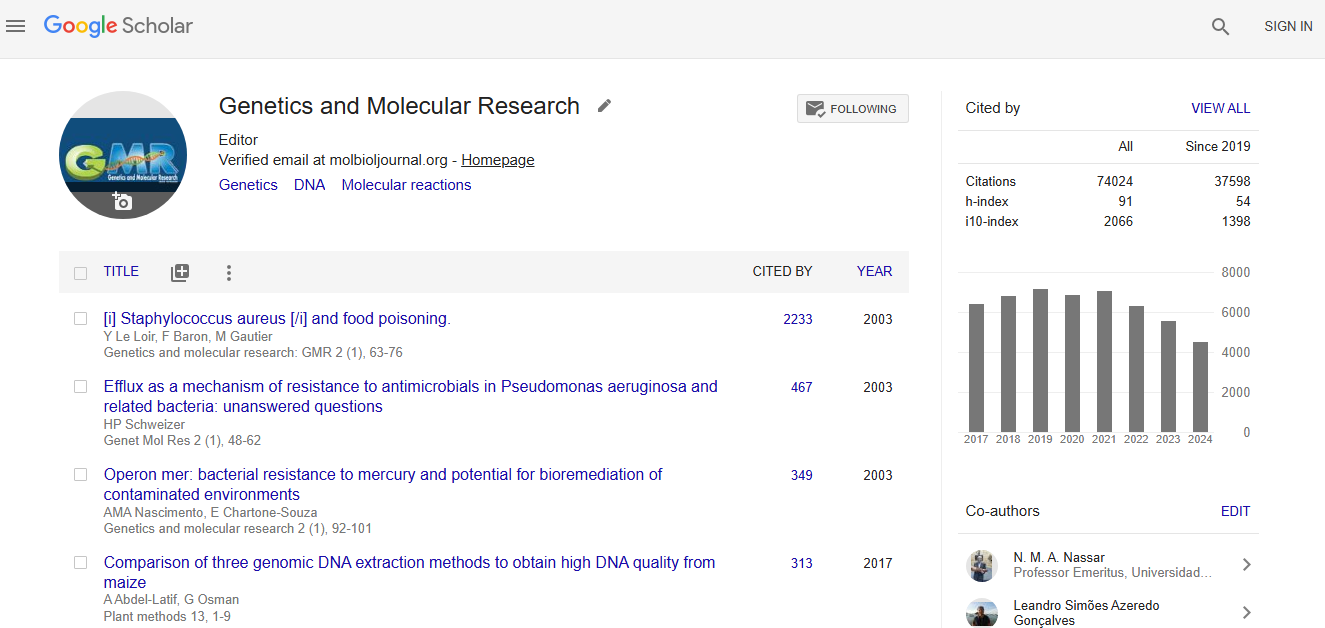
Google scholar citation report
Citations : 74024
Genetics and Molecular Research received 74024 citations as per google scholar report