Abstract
Bortezomib-based treatment of acute antibody-mediated rejection: a case report
Author(s): Q. Wang, X.L. Li, X.G. Xu, B.Y. Shi, Z.M. Zhang, Z.L. Li, Y. Han, W.Q. Zhou, C.Q. Chen, M. Cai and X. ZhangAntibody-mediated rejection (AMR) is an important factor affecting survival after renal transplantation. A highly selective proteasome inhibitor, bortezomib, clears activated plasma cells from the body and has important therapeutic effect on AMR. We investigated the effects of bortezomib on AMR in a patient after a second renal transplant. Biopsy confirmed the diagnosis of mixed cellular rejection and AMR. Bortezomib was administered on day 1 (1.3 mg/m2), day 4 (1.0 mg/m2), and day 8 (1.0 mg/m2). On the same days, 250 mg methylprednisolone was administered once, and cyclosporine dose (5 mg·kg-1·day-1) was reduced by 50%. Oral mycophenolate mofetil and steroid were withdrawn on day 1 of bortezomib treatment. Intermittent double-filtration plasmapheresis was also performed. We monitored parameters, including T lymphocyte subsets, CD139 and CD19 expression, panel reactive antibody (PRA), and serum creatinine concentration. At follow-up 6 months after bortezomib treatment, we observed: 1) serum creatinine stabilized at 130 μM from a peak level of 337 μM; 2) PRA decreased from a maximum of 66.7 to 0%; 3) blood plasma cell percentage rebounded after significantly decreasing following the first dose of bortezomib; 4) in renal allograft biopsy, immunohistochemical staining for C4d shifted from strongly positive to negative, and cellular rejection shifted from type IIA to borderline; and 5) adverse effects such as platelet suppression, hypotension, and grade 3 peripheral neuropathy emerged. Bortezomib effectively treated antibody-mediated renal transplantation rejection in this case study, but clinical trials with large sample sizes are still needed to explore clinical safety and tolerability.
Impact Factor an Index

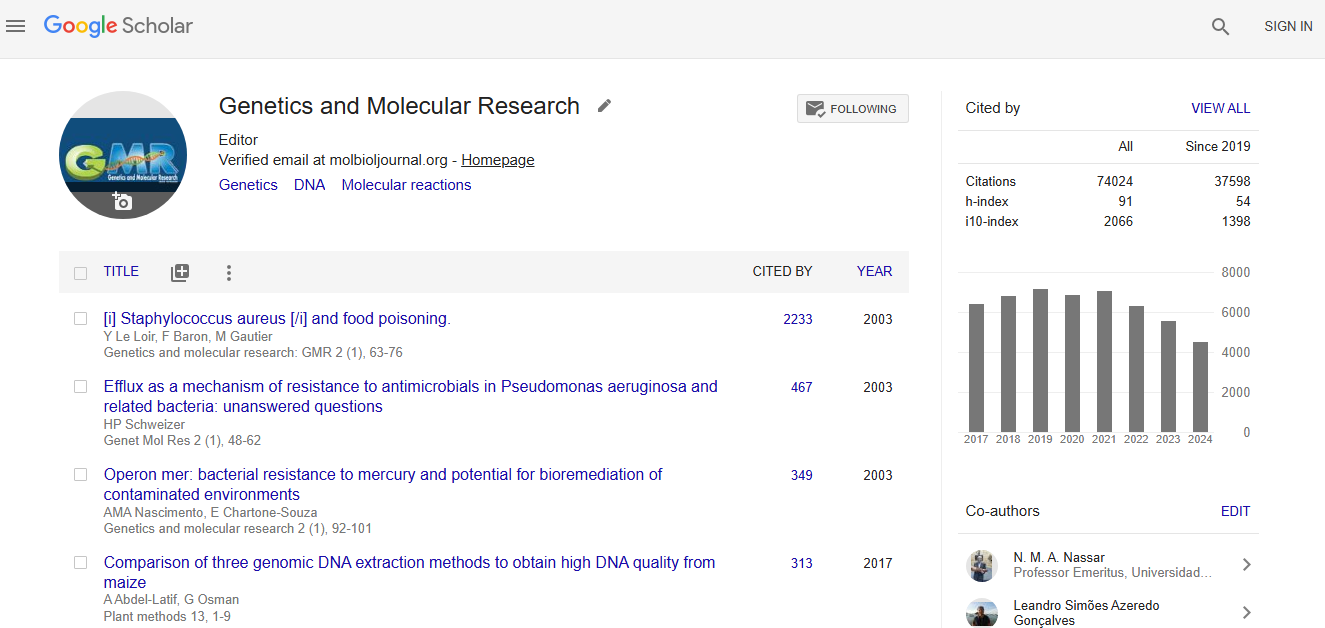
Google scholar citation report
Citations : 74024
Genetics and Molecular Research received 74024 citations as per google scholar report