Abstract
Avian sarcoma and leukosis virus gag gene - Genet. Mol. Res. 14 (4): 14379-14386 ��?Avian sarcoma and leukosis virus gag gene in the Anser anser domesticus genome�
Author(s): D. Elleder and J. HejnarDear Editor, A recent paper in the GMR Journal (Zhu et al., 2015) reported the discovery of endogenous avian sarcoma and leukosis virus (enASLV) in the domestic goose (Anser anser domesticus) genome. The discovery was based on PCR detection of a single viral gene (gag). This would be a very surprising finding, as ASLV-related endogenous retroviruses have only been detected in galliform birds (Dimcheff et al., 2000). Geese, which belong to the order Anseriformes, split from the Galliformes more than 80 million years ago. We argue below that the data supporting the findings by Zhu et al. are technically unsatisfactory, and that much stronger evidence would be needed. In our view, it is possible that their PCR amplifications were contaminated with chicken genomic DNA. Zhu et al. used PCR to amplify an approximately 1200-nucleotide fragment of the enASLV from all the eight Chinese domestic goose breeds that they tested. They used primers, designed in a previous study (Dimcheff et al., 2000), complementary to conserved regions of the ASLV gag gene. The sequences obtained by Zhu et al. were more than 99% identical to sequences present in the chicken genome. The authors used three arbitrarily chosen chicken enASLVs to analyze the phylogenetic relationship between the purported goose enASLV and the chicken enASLV. They concluded that there is a clear separation between chicken and goose sequences and that the goose sequences are more variable than the chicken sequences. However, we analyzed the purported goose ASLV sequence against the complete set of more than a hundred enASLVs assembled in a previous study (Dimcheff et al., 2000). In that study, the ASLV gag genes were amplified from 26 species of galliform birds. The phylogenies of viruses and avian hosts were largely congruent, indicating long association and vertical transmission during speciation of Galliformes. In our phylogenetic analysis, the purported goose ASLV sequence falls deeply within the cluster of chicken gag sequences (Figure 1). This indicates that the purported goose ASLV reported by Zhu et al. would have to have been formed by a very rare horizontal transmission event, most likely from chicken to goose. Nevertheless, drawing conclusions based on a comparison with only three chicken sequences without taking into account the full variability of known enASLVs is misleading. For control purposes, we attempted to experimentally verify the results of Zhu et al. using the same primers directed against ASLV gag. We failed to obtain any PCR products from both the domestic goose and the swan goose (Anser cygnoides). Zhu et al. used an excessive amount of genomic DNA template in their PCR amplification (0.5-1.0 μg in 20-μL PCRs), which makes it even more susceptible to contamination. Zhu et al. also confuse the claim that the goose genome contains endogenous retroviruses (ERVs) in general with the claim that it specifically contains endogenous ASLV, which is a subtype of endogenous alpharetroviruses. However, virtually all vertebrate genomes sequenced so far contain various types of ERVs. For example, they were found in 48 recently sequenced bird genomes (Cui et al., 2014). Still, enASLVs have hitherto been detected only in Galliformes. Indeed, Dimcheff et al. failed to amplify ASLV sequences from other birds, including Anseriformes. Zhu et al. refer to a study (Gifford et al., 2005) that identified class II-related ERV in the white-fronted goose (Anser albifrons). Although ASLVs do belong to class II ERVs, the white-fronted goose sequence reported is very distant (approximately 48% identity at amino acid level) from ASLVs and is more closely related to betaretroviruses. In addition, an attempt (Grunder et al., 1993) to identify ASLV-related viruses in the goose genome by Southern blotting and hybridization with a virus probe was negative; it included both the domestic goose and the swan goose. Zhu et al. mention these negative results but argue that hybridization is a low sensitivity technique compared with PCR and therefore might miss the purported goose ASLV. However, sensitivity is not an issue in the detection of ERVs, because unlike exogenous viruses, ERVs are always present in at least one copy per every cellular genome equivalent. An important consideration is specificity in the detection of closely related sequences. However, in the study, Grunder et al. used low-stringency conditions that can obtain a signal at 80% or greater homology. Therefore, they would definitely have detected the purported goose sequences (>99% homology to ASLV) if they had been present. Also worth mentioning is the fact that the swan goose genome sequence has already been published (Lu et al., 2015). Although this genome contains various classes of retroelements including ERVs, our BLAST searches using ASLV sequences as probes failed to recover any related elements with even close homology (BLAST e-value Dear Editor, A recent paper in the GMR Journal (Zhu et al., 2015) reported the discovery of endogenous avian sarcoma and leukosis virus (enASLV) in the domestic goose (Anser anser domesticus) genome. The discovery was based on PCR detection of a single viral gene (gag). This would be a very surprising finding, as ASLV-related endogenous retroviruses have only been detected in galliform birds (Dimcheff et al., 2000). Geese, which belong to the order Anseriformes, split from the Galliformes more than 80 million years ago. We argue below that the data supporting the findings by Zhu et al. are technically unsatisfactory, and that much stronger evidence would be needed. In our view, it is possible that their PCR amplifications were contaminated with chicken genomic DNA. Zhu et al. used PCR to amplify an approximately 1200-nucleotide fragment of the enASLV from all the eight Chinese domestic goose breeds that they tested. They used primers, designed in a previous study (Dimcheff et al., 2000), complementary to conserved regions of the ASLV gag gene. The sequences obtained by Zhu et al. were more than 99% identical to sequences present in the chicken genome. The authors used three arbitrarily chosen chicken enASLVs to analyze the phylogenetic relationship between the purported goose enASLV and the chicken enASLV. They concluded that there is a clear separation between chicken and goose sequences and that the goose sequences are more variable than the chicken sequences. However, we analyzed the purported goose ASLV sequence against the complete set of more than a hundred enASLVs assembled in a previous study (Dimcheff et al., 2000). In that study, the ASLV gag genes were amplified from 26 species of galliform birds. The phylogenies of viruses and avian hosts were largely congruent, indicating long association and vertical transmission during speciation of Galliformes. In our phylogenetic analysis, the purported goose ASLV sequence falls deeply within the cluster of chicken gag sequences (Figure 1). This indicates that the purported goose ASLV reported by Zhu et al. would have to have been formed by a very rare horizontal transmission event, most likely from chicken to goose. Nevertheless, drawing conclusions based on a comparison with only three chicken sequences without taking into account the full variability of known enASLVs is misleading. For control purposes, we attempted to experimentally verify the results of Zhu et al. using the same primers directed against ASLV gag. We failed to obtain any PCR products from both the domestic goose and the swan goose (Anser cygnoides). Zhu et al. used an excessive amount of genomic DNA template in their PCR amplification (0.5-1.0 μg in 20-μL PCRs), which makes it even more susceptible to contamination. Zhu et al. also confuse the claim that the goose genome contains endogenous retroviruses (ERVs) in general with the claim that it specifically contains endogenous ASLV, which is a subtype of endogenous alpharetroviruses. However, virtually all vertebrate genomes sequenced so far contain various types of ERVs. For example, they were found in 48 recently sequenced bird genomes (Cui et al., 2014). Still, enASLVs have hitherto been detected only in Galliformes. Indeed, Dimcheff et al. failed to amplify ASLV sequences from other birds, including Anseriformes. Zhu et al. refer to a study (Gifford et al., 2005) that identified class II-related ERV in the white-fronted goose (Anser albifrons). Although ASLVs do belong to class II ERVs, the white-fronted goose sequence reported is very distant (approximately 48% identity at amino acid level) from ASLVs and is more closely related to betaretroviruses. In addition, an attempt (Grunder et al., 1993) to identify ASLV-related viruses in the goose genome by Southern blotting and hybridization with a virus probe was negative; it included both the domestic goose and the swan goose. Zhu et al. mention these negative results but argue that hybridization is a low sensitivity technique compared with PCR and therefore might miss the purported goose ASLV. However, sensitivity is not an issue in the detection of ERVs, because unlike exogenous viruses, ERVs are always present in at least one copy per every cellular genome equivalent. An important consideration is specificity in the detection of closely related sequences. However, in the study, Grunder et al. used low-stringency conditions that can obtain a signal at 80% or greater homology. Therefore, they would definitely have detected the purported goose sequences (>99% homology to ASLV) if they had been present. Also worth mentioning is the fact that the swan goose genome sequence has already been published (Lu et al., 2015). Although this genome contains various classes of retroelements including ERVs, our BLAST searches using ASLV sequences as probes failed to recover any related elements with even close homology (BLAST e-value
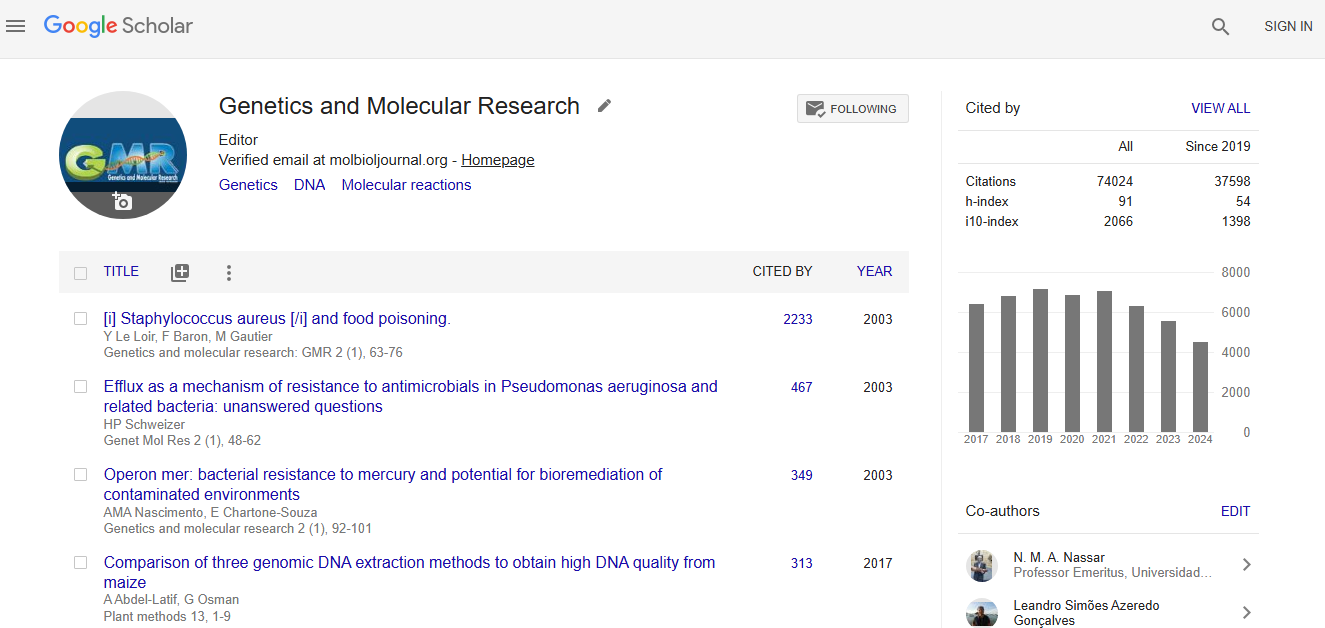
Impact Factor an Index

Google scholar citation report
Citations : 74024
Genetics and Molecular Research received 74024 citations as per google scholar report